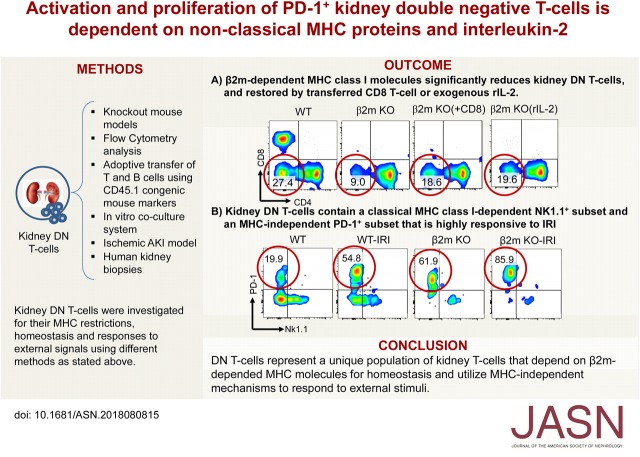
Significance Statement
Understanding how each immune cell type functions in the kidney is necessary to develop new immunotherapies for cell-mediated kidney diseases, including transplant rejection, AKI, and GN. Although CD4− CD8− double-negative (DN) T cells are a significant kidney T cell subpopulation that is anti-inflammatory and protective during ischemic AKI, little is known about the role of various MHC class I and II molecules in regulating their function. In addition to showing that mouse kidney DN T cells have IL-2–dependent proliferation, the authors identified two subsets of kidney DNT cells: a classical MHC class I–dependent NK1.1+ subset and an MHC-independent programmed cell death protein-1 receptor (PD-1+) subset that is highly responsive to ischemia-reperfusion injury. The presence of both subsets in human kidneys suggests that these findings are clinically relevant.
Keywords: immunology, ischemia-reperfusion, T cells
Visual Abstract
Abstract
Background
CD4− CD8− double-negative (DN) αβ T cells with innate-like properties represent a significant component of T cells in human and mouse kidneys. They spontaneously proliferate in the steady state and protect against ischemic AKI. However, the mechanisms regulating DN T cell homeostasis and responses to external danger signals from “sterile” inflammation remain poorly understood.
Methods
We used knockout mice, functional assays, and an established ischemic AKI model to investigate the role of various MHC class I and II molecules in regulating kidney DN T cells. We also studied human nephrectomy samples.
Results
Deficiency of β2m-dependent MHC class I (but not MHC class II) molecules led to significant reduction in frequency or absolute numbers of kidney DN T cells due to impaired activation, proliferation, increased apoptosis, and loss of an NK1.1+ subset of DN T cells. The remaining DN T cells in β2m knockout mice mainly comprised a programmed cell death protein-1 receptor (PD-1+) subset that depends on IL-2 provided by conventional T cells for optimal homeostasis. However, this PD-1+ subset remained highly responsive to changes in milieu, demonstrated by responses to infused lymphocytes. It was also the major responder to ischemic AKI; the NK1.1+ subset and CD8+ T cells had minimal responses. We found both DN T cell subsets in normal and cancerous human kidneys, indicating possible clinical relevance.
Conclusions
DN T cells, a unique population of kidney T cells, depend on nonclassical β2m molecules for homeostasis and use MHC-independent mechanisms to respond to external stimuli. These results have important implications for understanding the role these cells play during AKI and other immune cell–mediated kidney diseases.
The kidney is the most transplanted solid organ worldwide1 and is often an autoimmune target. Thus, significant efforts are being directed toward a better understanding of the immune cell types in the kidney, their relations to one another as well as how they are modified by local microenvironments and external stimuli. This information is vital for understanding and treatment of diseases ranging from transplant rejection and AKI to glomerulonephritis (GN) and renal cell cancer.2,3
Analysis of kidney resident T cells has traditionally focused on conventional CD4 and CD8 T cells.4 Besides conventional T cells, CD4− CD8− double-negative (DN) αβ T cells represent a significant component (approximately 25%) of kidney T cells that remains poorly understood.5 Recent results from our group and others show that kidney DN T cells are major responders to ischemic AKI as they undergo rapid expansion (within 24 hours) after ischemia. In addition, kidney DN T cells have in vitro suppressive function against conventional T cells and transfer of DN T cells isolated from lymph nodes of gld mice protect against AKI.6
DN T cells reside in other nonlymphoid organs (e.g., lung, liver),5 including gut epithelium.7 DN T cells have a strong immunologic response against bacteria and viruses in mice, implicating their immunologic functions in the host defense mechanism. In addition, DN T cells have been implicated in disease pathogenesis, for example, in Behcet disease and lupus nephritis.8
There are many important unknowns about kidney DN T cells. For example, understanding MHC restriction element of each cell type is important to predict their ligands, the immune cell types they interact with, and for devising strategies to regulate their homeostasis.9 MHC class I presents endogenous, but not exogenous, peptides to CD8 T cells, whereas MHC class II presents mainly exogenous peptides to CD4 T cells. In certain circumstances, internalized foreign antigens are presented by MHC class I molecules by a process that is referred to as cross presentation. Most, but not all, nonclassic MHC molecules depend on β2m molecules for their surface expression. The nonclassic MHC that are β2m-dependent include CD1d molecules present lipid antigens to NKT cells10 and MR1 molecules present vitamin B precursors and metabolites to MAIT cells.11 The few nonclassic MHC that are β2m-independent include EPCR, and ZAG, which present lipids, peptides, and fatty acids, respectively. However, the antigen presentation of other molecules like MILL and RAE1 have not been well studied.12
In this study, we demonstrate that DN T cells represent a unique population of kidney T cells that depends on nonclassic β2m molecules for their homeostasis and utilizes MHC-independent mechanisms to respond to external stimuli. We identify two major subsets of kidney DN T cells, programmed cell death protein-1 receptor (PD-1+) and NK1.1+, both in mice and humans. We also elucidate an important role for IL-2 in DN T cell regulation. Furthermore, we examine DN T cell responses to “sterile” inflammation from ischemic AKI. These results will have important implications for understanding the role of DN T cells in a broad spectrum of immune cell–mediated kidney diseases.
Methods
Mice
C57BL/6J (wild type; WT), B6.129P2-B2mtm1Unc/J (β2m knockout; KO), B6.129S2-H2dlAb1-Ea/J (MHC II KO), B6.129P2-H2-K1tm1Bpe H2-D1tm1Bpe/DcrJ (KD KO), and B6.SJL-Ptprca Pepcb/BoyJ (CD45.1) mice were purchased from Jackson Laboratory. All mice were kept and bred under specific pathogen-free conditions at the Animal Facility of Johns Hopkins University. Age-matched mice between the age of 8 and 10 weeks were used in the study, unless stated otherwise. All experiments were performed using experimental protocols approved by the Animal Care and Use Committee of Johns Hopkins University.
Antibodies
Fluorochrome-conjugated mAbs were purchased from BD Biosciences, BioLegend, or eBioscience for flow cytometry analysis. We used the following mAbs to mouse antigens: CD45–APC-Cy7 (30-F11), αβTCR-Pacific blue (H57–597), CD8α-PerCP (53–6.7), CD4-APC (RM4–5), CD28-PE (37.51), CD69-FITC (1H.2F3), CD62L-PECy7 (MEL-14), CD44-PE (IM7), Ki-67–PerCP–eFluor 710 (SolA15), Annexin V-PE, CD45.1-PE (A20), CD45.2–APC-Cy7 (104), CD25-PE (PC61), CD45R/B220-PE (RA3–6B2), Thy1.2-PE (53–2.1), CD178-PE (MFL3), CD95-FITC (Jo2), IL-2–Pacific blue (JES6–5H4), NK1.1-FITC (PK136), and PD-1-BV421 (J43). We used the following mAbs to human antigens: CD45–APC-Cy7 (HI30), αβTCR-FITC (IP26), CD8α–PE-Cy7 (HIT8a), CD4-APC (OKT4), NK1.1-PE (HP-3G10), and PD-1–Pacific blue (EH12.2H7). Mouse CD1d tetramers were from the National Institutes of Health tetramer core facility.
Isolation of Lymphocytes from Kidneys
Mononuclear cells from kidneys were isolated as previously described.13 Briefly, kidneys were minced and incubated in collagenase D (5 mg/ml; Sigma-Aldrich, St. Louis, MO) solution for 30 minutes at 37°C. Single-cell suspensions of kidney digestions were obtained by mechanical disruption of tissues using 70-μm strainers (BD Bioscience). The absolute numbers of viable lymphocytes in each sample was determined using the trypan blue exclusion dye in a hemocytometer under light microscope and absolute numbers of each population determined by multiplying total cell number per organ by percentage of the specific population as determined in FACS dot plots.
Flow Cytometry Analysis and Gating Strategy
Flow cytometric analysis was performed using standard methods.14 Briefly, cells were stained in FACS buffer (PBS, 2% FBS, 0.1% sodium azide) and preincubated with anti-CD16/CD32 antibody for 10 minutes to minimize nonspecific binding through Fc-receptors. Cells were incubated with appropriate cocktails of fluorochrome-conjugated mAbs for 30 minutes at 4°C, washed, and resuspended in FACS buffer. Samples were acquired using a BD LSR II flow cytometer with four lasers line colors (405, 488, 561, and 640 nm). Data were analyzed using the FlowJo V10 software (Treestar Software).
Ki-67 Proliferation Assay
Proliferation of T cells was examined by analysis of intracellular expression of the Ki-67 antigen using anti–Ki-67 mAb. Lymphocytes from different organs were isolated and stained for surface markers, followed by intracellular staining of Ki-67 with Foxp3 staining buffer (eBioscience). Samples were acquired by flow cytometer and analyzed by FlowJo.
Annexin V Apoptosis Assay
Apoptosis was assessed using PE-Annexin V apoptosis kit according to manufacturer’s instructions (BD Pharmingen). Cells were stained for surface markers for 30 minutes at 4°C, washed twice with FACS buffer, resuspended in Annexin V binding buffer with anti-Annexin mAb for 15 minutes in dark at room temperature, acquired by flow cytometer, and analyzed by FlowJo.
Adoptive Transfer of T and B Cells
Highly purified CD8, CD4 T cells or B cells (>97%) were isolated from WT spleens using Pan CD8, CD4 T cells or B cells Isolation Kit (Miltenyi Biotec) and used for adoptive transfer. β2m KO mice in experimental groups were intraperitoneally injected with indicated population of lymphocytes (5×106), whereas age-matched mice in control groups were injected with PBS. Five days after transfer, lymphocytes were isolated from kidneys, stained, acquired by LSRII, and analyzed by FlowJo. When relevant, CD45.1 and CD45.2 markers were used to distinguish between donor and host lymphocytes.
In Vitro Coculture Analysis of DN T Cells
We used sorted T or B cells in these experiments to ensure purity (>99%). Unless otherwise stated, DN T cells were sorted from kidneys (1.2 to 1.8×105 DN T cells per sort from six mice) and T or B cells from spleens of WT donors using established procedures.14 Briefly, sorted DN T cells, and CD8 or CD4 T cells or B cells (2×104 cells per well used for surface staining or 5×104 cells per well used for intracellular staining) were cultured at 1:1 ratio or separately, in complete tissue culture media (ThermoFisher, Waltham, MA) containing 10% FBS and 100 U/ml penicillin and streptomycin. Cells were stimulated with plate-bound anti-CD3 (10 µg/ml) and anti-CD28 (10 µg/ml) for 5 days, as previously described.15 For IL-2 neutralization, anti–IL-2 (JES6–5H4) (10 µg/ml) was used. Cells were harvested, counted, and stained using appropriate cocktails of fluorochrome-conjugated mAbs, acquired by LSRII and percentage of each subpopulation determined using FlowJo. The absolute cell numbers of each subset (DN and CD8 or CD4 T cells or B cells) were calculated.
Cytometric Bead Array Mouse Th1/Th2/Th17 Cytokine Kit Assay
Briefly, culture supernatants from single or coculture was used to determine cytokines secreted in cultures using the CBA assay kit (BD Biosciences) according to manufacturer’s instruction (BD Biosciences).16
IL-2/Anti–IL-2 mAbs Complex Treatment
IL-2/anti–IL-2 mAb complexes (IL-2 CMPLX) were delivered to β2m KO mice by intraperitoneal injection of 1.5 μg of recombinant mouse IL-2 (ProSpec, East Brunswick, NJ) premixed (for 18 hours) with 7.5 μg of anti–IL-2 mAb (JES6–5H4).17 Lymphocytes were isolated from kidneys after 48 hours of IL-2 CMPLX transfer, stained, acquired by flow cytometer, and analyzed by FlowJo.
Kidney Ischemia-Reperfusion Injury Model
An established model of bilateral renal ischemia-reperfusion in mice was used as previously described.18 Briefly, mice were anesthetized with an intraperitoneal injection of ketamine and xylazine (at a ratio of 130:7 mg/kg). After an abdominal medial incision, both renal pedicles were dissected, and a microvascular clamp (Roboz Surgical Instrument, Gaithersburg, MD) was placed on each renal pedicle for 30 minutes. Animals were kept well hydrated with warm saline and at constant body temperature (37°C). After 30 minutes of ischemia, the clamps were removed and wounds were sutured. The animals were allowed to recover with free access to food and water. Serum creatinine levels were determined at baseline and 24 hours after renal ischemia to confirm the injury. Lymphocytes were isolated after 24 hours of renal ischemia, from kidneys and lymphoid organs, and then stained, acquired by LSRII, and analyzed by FlowJo.
Statistical Analyses
Data were collected from at least three independent experiments and expressed as mean±SEM. n indicates the number of animals per group. Unpaired t tests were used for comparison of repeated measures in the same group. Comparisons between multiple groups were performed by a one-way or two-way ANOVA test followed by the Tukey or Sidak multiple comparison test where appropriate. Statistical analysis was performed using Prism 6, GraphPad Software, and significance was determined as P<0.05.
Results
β2m-Dependent Nonclassic MHC1b Molecules Regulate Homeostasis of Kidney DN T Cells
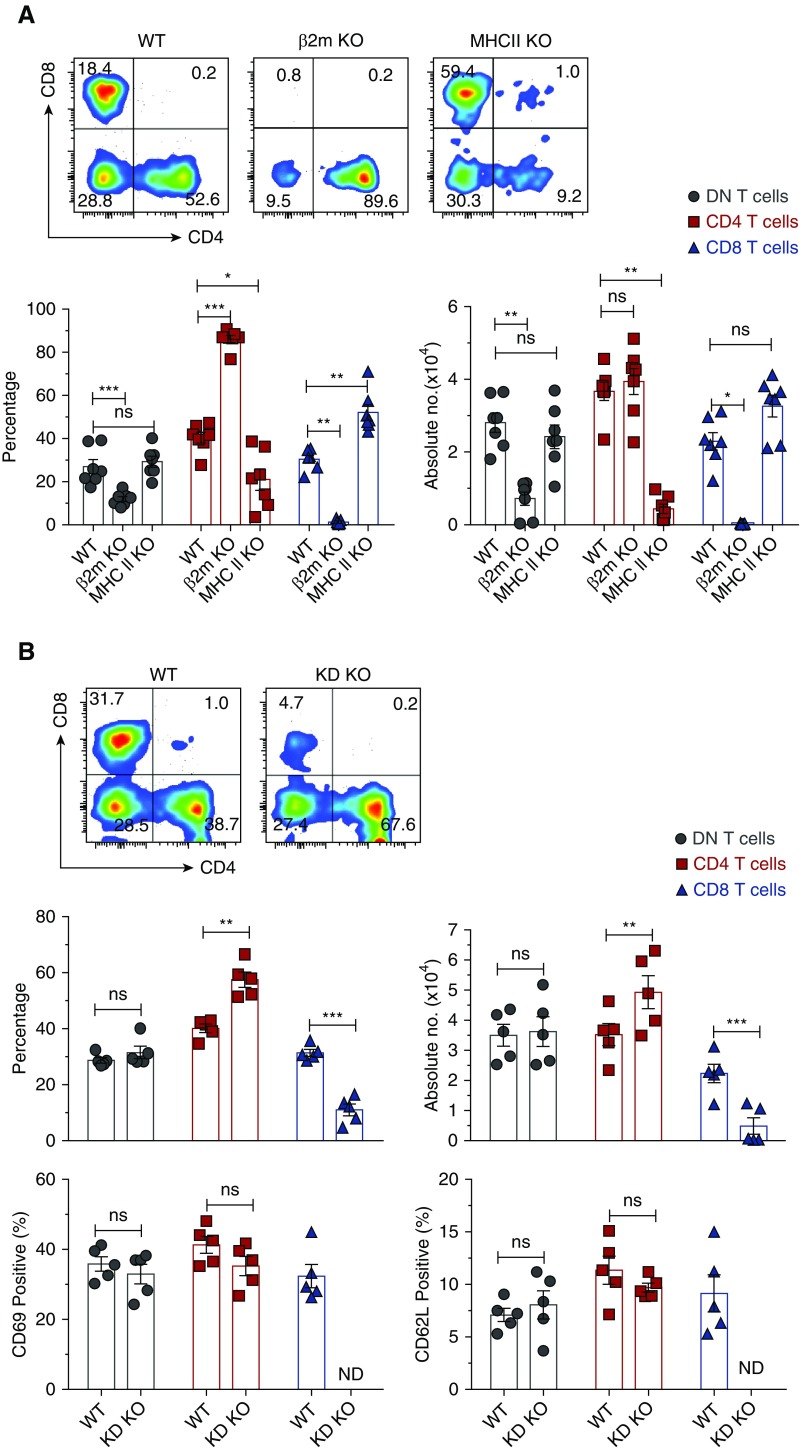
To identify roles of MHC class I and II in regulating homeostasis of kidney DN T cells, we analyzed effects of the absence of β2m or MHC class II molecules on kidney DN T cells. Kidney CD4, CD8, and DN T cell subsets were identified as depicted in our gating strategy (Supplemental Figure 1). Percentage and the absolute number of DN T cells were significantly decreased in kidneys and thymi, but not lymph nodes, of β2m KO mice (Figure 1A and Supplemental Figure 2). Furthermore, β2m deficiency did not or minimally affected DN T cells in other nonlymphoid organs, such as heart (WT, 8.8%±2.1% versus β2m KO, 8.7%±1.7%) and liver (WT, 12.0%±1.9% versus β2m KO, 10.6%±3.2%). As expected, CD8 T cells were virtually absent in kidneys of β2m KO mice, and therefore not investigated further in these mice. Furthermore, there is no significant defect in frequency or absolute numbers of DN T cells in kidney, thymus, or lymph nodes of mice lacking MHC II (Figure 1A and Supplemental Figure 2).
Figure 1.
β2m-dependent MHC1b, but not MHC II or MHC1a, molecules significantly reduce relative and absolute number of kidney DN T cells. Lymphocytes from kidneys of age-matched WT, β2m KO, MHC II KO, or KD KO mice were isolated, stained, and acquired by LSRII and CD4, CD8, and DN αβ T cell subsets were gated as described (Supplemental Figure 1). (A) Dot plots show representative percentages of CD4, CD8, and DN T cells in kidneys of WT, β2m KO, and MHC II KO mice. Numbers in quadrants indicate percentages. Graphs show cumulative frequency and absolute numbers of T cells in kidney. Data points (mean±SEM) are from at least seven independent experiments (n=7 mice). (B) Dot plots show representative percentages of CD4, CD8, and DN T cells in kidneys of WT and KD KO mice. Numbers in quadrants indicate percentages. Graphs show cumulative frequency, absolute numbers, and percentage of CD69 and CD62L of T cells in kidneys. Data points (mean±SEM) are from at least five independent experiments (n=5 mice). *P<0.05; **P<0.01; ***P<0.001.
β2m is essential for the expression of classic MHC class 1a (K and D) and most nonclassic MHC class 1b (MHC 1b) and MHC-like molecules (CD1, MR1).12 Hence, the negative effect of β2m deficiency on kidney DN T cells could be due to the lack of MHC 1a, MHC 1b, or both. To distinguish between these possibilities, we analyzed DN T cells in the kidney of KD KO mice that lacked classic H2-K and H2-D molecules, but not MHC 1b molecules.19 The frequencies and absolute numbers of kidney DN T cells as well as their activation state in KD KO mice were comparable with those in age-matched WT mice, indicating that they are not regulated by MHC 1a (Figure 1B). There were also no detectable alterations in the activation state of kidney CD4 T cells of KD KO mice, except for increases in their percentage and absolute numbers due to the loss of CD8 T cells (Figure 1B). These results exclude a major role for MHC 1a or MHC II in the development or homeostasis of kidney DN T cells, indicating that they are regulated primarily by β2m-dependent MHC 1b molecules.
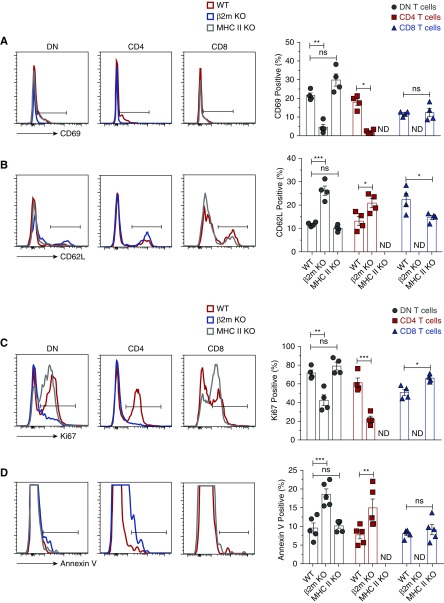
β2m Deficiency Impairs Activation, Proliferation, and Increased Apoptosis of Kidney DN T Cells in the Steady State
Kidney DN T cells have preactivated phenotypes as determined by the high expression of CD69 and downregulation of CD62L. However, β2m deficiency significantly impaired activation of DN T cells in the kidney of β2M KO mice as indicated by significant decrease in surface expression of CD69 and increase of CD62L (Figure 2, A and B), compared with WT controls. Nevertheless, DN T cells maintained high levels of surface CD44 in β2m KO mice, as did their counterparts in WT controls (Supplemental Figure 3). Expression of these activation markers, including CD44, were reduced in CD4 T cells (Figure 2, A and B and Supplemental Figure 3). It is currently unclear why kidney DN T cells of β2m deficiency maintained high expression of CD44, but it could be related to a role in regulating their homing20 and/or effectors functions.21 However, it is noteworthy that β2m deficiency did not alter surface expression of CD28 by DN T cells (Supplemental Figure 3). Furthermore, few kidney DN T cells expressed B220 (3.1%±0.5%) or Thy 1.2 (1.5%±0.7%), whereas some expressed Fas or FasL (Supplemental Figure 4). Thus, kidney DN T cells are different from peripheral DN T cells that cause lymphoproliferation in mice bearing loss-of-function point mutation in Fas (lpr) or FasL (gld). However, both gld DN T cells and kidney DN T cells have suppressive functions in vitro and are capable of ameliorating AKI in mice.6
Figure 2.
Lack of β2m, but not MHC II, impairs activation, proliferation, and increased apoptosis of kidney DN T cells in the steady state. Lymphocytes were isolated from kidneys of age-matched WT, β2m KO, or MHC II KO mice, stained, and acquired by LSRII. Gated CD4, CD8, and DN T cells were analyzed for the expression of CD69, CD62L, Ki-67, and Annexin V. Representative plots show percentage of (A) CD69, (B) CD62L, (C) Ki-67, and (D) Annexin V by each T cell type in the kidneys of individual genotype. Graphs show cumulative data from at least four or five independent experiments (n=4–5 mice). Data are expressed as mean±SEM. *P<0.05; **P<0.01; ***P<0.001.
The absolute number of T cells depends on proliferation and apoptosis rates. β2m deficiency significantly decreased proliferation and increased apoptosis of kidney DN T cells as determined using Ki-67 and Annexin V assays (Figure 2, C and D). β2m deficiency also decreased proliferation and increased apoptosis of kidney CD4 T cells. Consistent with the above results, deficiency of MHC II did not alter proliferation and apoptosis of kidney DN or CD8 T cells. Thus, decreased proliferation and increased apoptosis are at least partly responsible for the reduced numbers of kidney DN T cells in β2m KO mice.
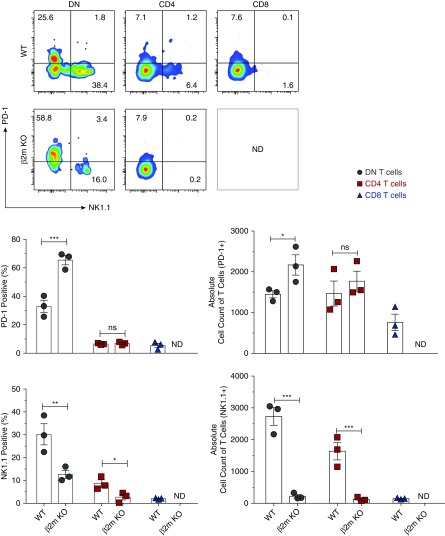
Kidney DN T Cells Comprise Two Subsets, NK1.1+ and PD-1+, and β2m Deficiency Leads to Selective Loss of the NK1.1+ Subset
It has recently been observed that DN T cells in murine thymus and gut epithelium contain two subsets, NK1.1+ and PD-1+.7 We found both subsets in mouse kidneys (Figure 3). β2m deficiency led to the loss of almost all kidney NK1.1+ DN T cells with remaining DN T cells virtually comprising the PD-1+ subset (Figure 3). We detected both subsets in the thymus and lymph nodes and the NK1.1+ subset was significantly reduced in the thymus, but not lymph nodes of β2m KO mice (Supplemental Figure 5). We also noted that some kidney CD4 and CD8 T cells expressed PD-1, whereas others expressed NK1.1. Taken together, our results show that the reduced number of kidney DN T cells in β2m-deficient mice is due to the loss of the NK1.1 subset and impaired proliferation and increased apoptosis of PD-1+ cells.
Figure 3.
β2m deficiency leads to loss of NK1.1+ DN T cells. Lymphocytes were isolated from kidneys of age-matched WT and β2m KO mice, stained, and acquired by LSRII. Gated CD4, CD8, and DN T cells were analyzed for the expression of PD-1 and NK1.1. Representative dot plots show percentage of PD-1+ or NK1.1+ subsets in mouse kidneys. Numbers in quadrants indicate percentages. Graphs show cumulative data. Data points (mean±SEM) are from at least three independent experiments (n=3 mice). *P<0.05; **P<0.01; ***P<0.001.
Transfer of CD8 or CD4 T Cells, but Not B Cells, Restores Homeostasis of Kidney DN T Cells in β2m KO Hosts
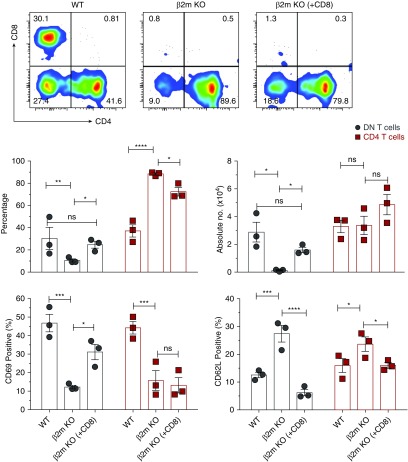
Next, we investigated why loss of the β2m molecule impairs activation and proliferation of PD-1+ DN T cells. One simple explanation is the loss of interaction of antigen(s) presented by β2m-dependent molecules. Alternatively, but not mutually exclusively, it could be secondary to the loss of CD8 T cells. To distinguish between these possibilities, we determined whether transfer of WT CD8 T cells restores proliferation of kidney DN T cells in β2m KO mice. Transfer of CD8 T cells led to significant increases in frequency and absolute numbers of kidney DN T cells accompanied by increased activation as indicated by upregulation of CD69 and downregulation of CD62L (Figure 4). There were also no detectable alterations in the numbers and activation of kidney CD4 T cells after transfer of CD8 T cells. To rule out the latter possibility, we used CD45.1 and CD45.2 congenic markers to distinguish between donor and recipient DN T cells. Almost all expanded kidney DN T cells expressed CD45.2, thereby excluding the possibility that expanded DN T cells were derived from donor CD8 T cells that downregulated their CD8 coreceptor22 (Supplemental Figure 6). Thus, reconstitution of CD8 T cells was sufficient to restore homeostasis of DN T cells in β2m KO mice.
Figure 4.
Adoptive transfer of WT CD8 T cells into β2m KO mice significantly increases absolute numbers and activates endogenous DN T cells. Purified CD8 T cells from WT spleens were intraperitoneally injected (5×106 cells per mouse) into β2m KO mice, whereas age-matched WT controls received PBS. Five days later, the frequency and absolute numbers of CD4 and DN T cells in kidneys were analyzed by flow cytometry. Representative dot plots show percentages of CD4, CD8, and DN T cells in kidneys of WT control, β2m KO, and β2m KO mice injected with CD8 T cells [β2m KO (+CD8)]. Numbers in quadrants indicate percentages. Top graphs show cumulative percentages and absolute numbers of each subsets in indicated organs of mice in each group. Bottom graphs show frequency of CD69 and CD62L on gated CD4 and DN T cell subsets in kidneys of mice in the three different groups. Data points expressed as mean±SEM are from at least three independent experiments (n=3 mice). *P<0.05; **P<0.01; ***P<0.001; ****P<0.001.
Besides provision of CD8 T cells themselves, the reconstitution provided a source of β2m-dependent molecules. To distinguish between these possibilities, we examined whether transfer of WT CD4 T cells or B cells has similar effects to that of CD8 T cells. Intriguingly, adoptively transferred CD4 T cells were also able to induce activation and expansion of kidney DN T cells, whereas transferred B cells caused activation (upregulation of CD69), but not expansion of DN T cells (Supplemental Figure 7), indicating that DN T cells had sensed but failed to proliferate in response to transferred B cells. Because both WT T and B cells express β2m molecules, the ability of T cells, but not B cells, to induce proliferation of kidney DN T cells is likely due to provision of factors/signals by T cells.
Provision of IL-2 by Conventional T Cells Is Necessary for Expansion of DN T Cells Both In Vitro and In Vivo
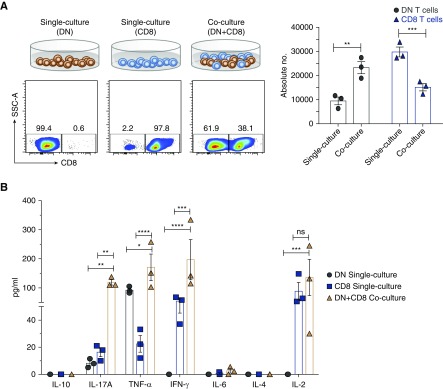
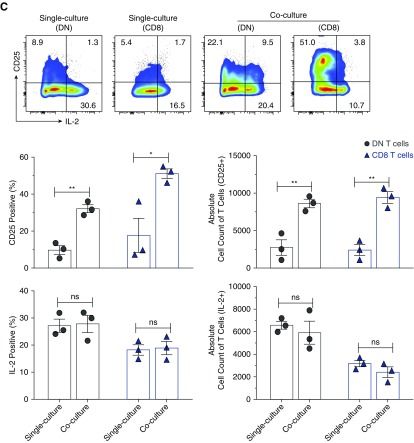
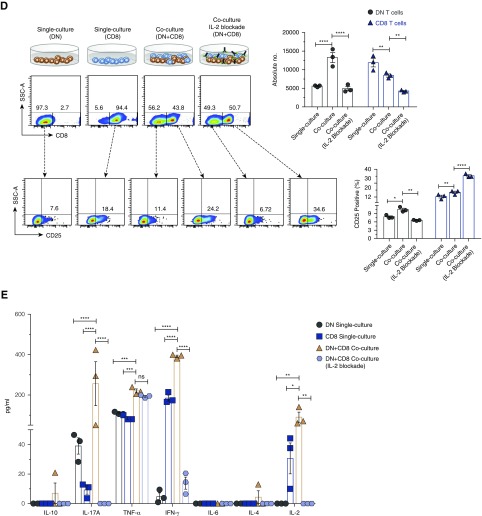
Next, we investigated how T cells induce proliferation of DN T cells using in vitro cocultures of sorted B cells, CD8, CD4, and DN T cells. Consistent with the in vivo results, both CD8 and CD4 T cells, but not B cells, were able to induce expansion of DN T cells in cocultures (Supplemental Figure 8). Subsequently, we focused on examining the role of CD8 T cells in activation of DN T cells. The coculturing of the two cell types led to significant expansion of DN T cells concomitant with suppression of CD8 T cells, compared with controls (Figure 5A). Furthermore, we detected significant increases of secreted IL-2, IL-17, IFN-γ, and TNF-α, but not IL-4, IL-6, or IL-10 in cocultures, compared with single cultures (Figure 5B). However, there was no significant increase of cytoplasmic IL-2 in DN T cells, but the percentage of CD25+ (IL-2Rα) DN T cells was significantly increased in cocultures (Figure 5C). Expression of CD25 enhances the ability of T cells to utilize IL-2 by 100-fold.23 Thus, the addition of CD8 T cells led to expansion of DN T cells and increased their ability to utilize IL-2. IL-2 production was important for DN T cell expansion because antibody blockade of IL-224 completely inhibited in vitro expansion of DN T cells with decreased CD25 expression (Figure 5D) and secretion of IL-2, and significantly decreased production of IL-17 and IFN-γ, but not TNF-α, in the cocultures (Figure 5E). IL-2 blockade also inhibited expansion of CD8 T cells, but not upregulation of surface CD25 (Figure 5D). Given that anti-CD3/CD28 activation did not lead to expansion of DN T cells in single culture, the results point to a major role for CD8 T cells in driving their expansion. These findings are relevant in vivo as well because transfer of CD8 T cells into β2m KO mice led to significant increase in surface expression of CD25 (approximately two-fold) and intracellular IL-2 (approximately 1.5-fold) by DN T cells (Supplemental Figure 9).
Figure 5.
Provision of IL-2 by CD8 T cells is necessary for expansion of DN T cells in vitro. Sorted kidney DN cells and splenic CD8 cells from WT mice (2×104 cells per well used for surface staining or 5×104 cells per well used for intracellular staining) were cultured together or separately and stimulated with immobilized anti-CD3/CD28 for 5 days before cells were harvested and analyzed for surface expression CD25 and intracellular IL-2, and supernatants examined for Th1/Th2/Th17 cytokines using cytometric bead array. (A) Dot plots show the representative flow data of kidney DN T cells and splenic CD8 T cells that were cultured together or separately. Numbers in quadrants indicate percentages. Graph shows absolute cell numbers of each T cell in single and mixed cultures. (B) Graph shows amounts of cytokines in culture supernatants from different cultures. (C) Dot plots show expression of CD25 and intracellular IL-2 by gated DN and CD8 T cells. Numbers in quadrants indicate percentages. Graphs show frequency and absolute cell numbers of CD25+ and IL-2+ DN or CD8 T cells. (D) Top dot plots show representative flow data of kidney DN T cells and splenic CD8 cells that were cultured together with or without anti-IL-2, or cultured separately. Numbers in quadrants indicate percentages. Top graph shows absolute cell numbers of each T cell in single and mixed cultures. Bottom dot plots show expression of CD25 by gated DN and CD8 T cells. Numbers in quadrants indicate percentages. Graphs show frequency of CD25+ DN or CD8 T cells. (E) Graph shows amounts of cytokines in culture supernatants from different cultures in the presence or absence of anti-IL2. Data points are shown for each mouse with mean ±SEM, from at least three independent experiments. *P<0.05; **P<0.01; ***P<0.001; ****P<0.001.
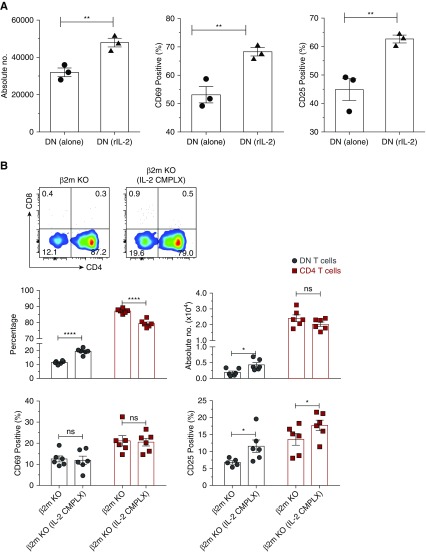
Exogenous IL-2 Is Sufficient to Expand DN T Cells in β2m KO Mice
The above results suggested that CD8 T cells promote proliferation possibly by provision of IL-2. We therefore determined whether addition of recombinant IL-2 (rIL-2) acts as a substitute for the role of CD8 T cells. We sorted kidney DN T cells from WT mice and cultured for 5 days in the presence or absence of rIL-2. Addition of rIL-2 significantly expanded DN T cells and upregulated surface CD69 and CD25 (Figure 6A). These in vitro observations translated to in vivo as injection of IL-2 complex (IL-2 CMPLX)17 into β2m KO mice significantly increased the frequency and absolute number of CD25, as well as surface CD25 on kidney DN T cells (Figure 6B). These results confirm that activation and proliferation of kidney DN T cells is dependent on IL-2 provided by other T cells.
Figure 6.
Exogenous IL-2 is sufficient to expand the DN T cells in β2m KO mice. (A) Sorted kidney DN T cells were cultured (2×104 cells per well) with or without rIL-2 (100 ng/ml). Five days later, cells were harvested, counted, and analyzed for the expression of CD69 and CD25 by flow cytometry. Graphs show the absolute numbers and percentage of CD69 and CD25 on cultured DN T cells in the presence or absence of rIL-2. Data points (mean±SEM) are from at least three independent experiments. (B) β2m KO mice were intraperitoneally injected with IL-2 (as IL-2 CMPLX), whereas age-matched β2m KO controls received PBS. Two days later, the frequency, absolute numbers, and percentage of CD69 and CD25 T cells in kidneys were analyzed by flow cytometry. Representative dot plots show percentages of CD4, CD8, and DN T cells in kidneys of β2m KO and β2m KO (IL-2 CMPLX) mice. Numbers in quadrants indicate percentages. Graphs show cumulative data. Data points (mean±SEM) are from at least six independent experiments (n=6 mice). *P<0.05; **P<0.01; ****P<0.001.
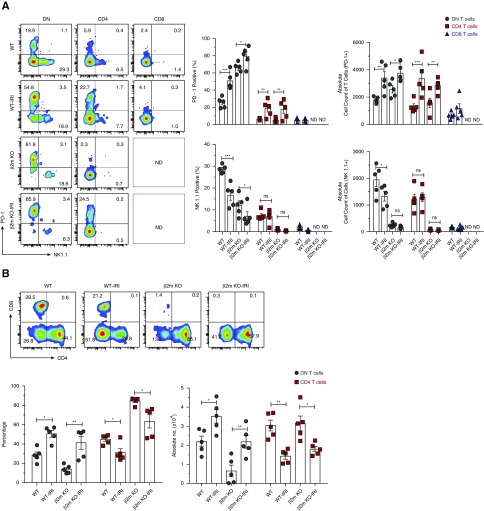
β2m-Independent PD-1+ DN T Cell Subset Is the Major Responder to Ischemic AKI, whereas the NK1.1+ DN T Cell Subset and CD8 T Cells Are Dispensable
Given our detection of the two subsets of DN T cells in the kidney, we sought to determine whether the two subsets respond differently to ischemia-reperfusion injury (IRI). Furthermore, development of CD8 T cells is dependent on expression of β2m-dependent MHC class I molecules,25 yet the effect of β2m deficiency on AKI has not been examined in murine models. Our results show that the PD-1+ subset is the major responder to IRI in WT mice, as indicated by significant expansion of PD-1+ cells at the expense of NK1.1+ cells (Figure 7A). In concordance, the PD-1+ subset predominated kidney DN T cells in ischemic kidneys of WT mice. Similar to PD-1+ DN T cells, PD-1+ CD4 T cells increased in response to IRI, but the NK1.1 subset was not significantly changed. In addition, we analyzed how DN T cells (mainly comprising PD-1+ cells) in β2m KO mice respond to IRI and overall susceptibility of β2m KO to AKI. We found that IRI led to significant expansion of DN T cells in β2m KO (Figure 7B), showing that kidney DN T cells remain functional and capable of rapid response to signals caused by IRI in the absence of β2m-dependent molecules. We did not observe any other significant effect of β2m deficiency on responses of mice to AKI compared with WT controls, at least within the first 24 hours after IRI, despite lacking CD8 T cells and NK1.1+ DN T cells. Serum creatinine levels increased significantly 24 hours after IRI in both WT (mean±SEM 2.1±0.07) and β2m KO mice (mean±SEM 2.2±0.10). These results exclude a major role for CD8 T cells and the NK1.1+ subset of DN T cells in the pathogenesis of AKI.
Figure 7.
β2m-independent PD-1+ DN T cell subset is the major responder to ischemic AKI whereas NK1.1+ DN T cell subset and CD8 T cells are dispensable. WT and β2m KO mice were subjected to IRI and serum creatinine levels were determined after 24 hours to confirm kidney injury. Lymphocytes were isolated from kidneys of WT, β2m KO, WT-IRI, and β2m KO-IRI mice and the frequency, absolute numbers, and percentage of PD-1+ or NK1.1+ subsets were analyzed by flow cytometry. (A) Representative dot plots show percentage of PD-1+ or NK1.1+ by gated T cell subsets in kidneys. Numbers in quadrants indicate percentages. Graph shows cumulative data. (B) Representative dot plots show T cell subsets from a kidney. Numbers in quadrants indicate percentages. Graphs show percentage and absolute numbers of T cells from kidneys from indicated mouse groups. Data points (mean±SEM) are from at least five independent experiments (n=5 mice). *P<0.05; **P<0.01; ***P<0.001.
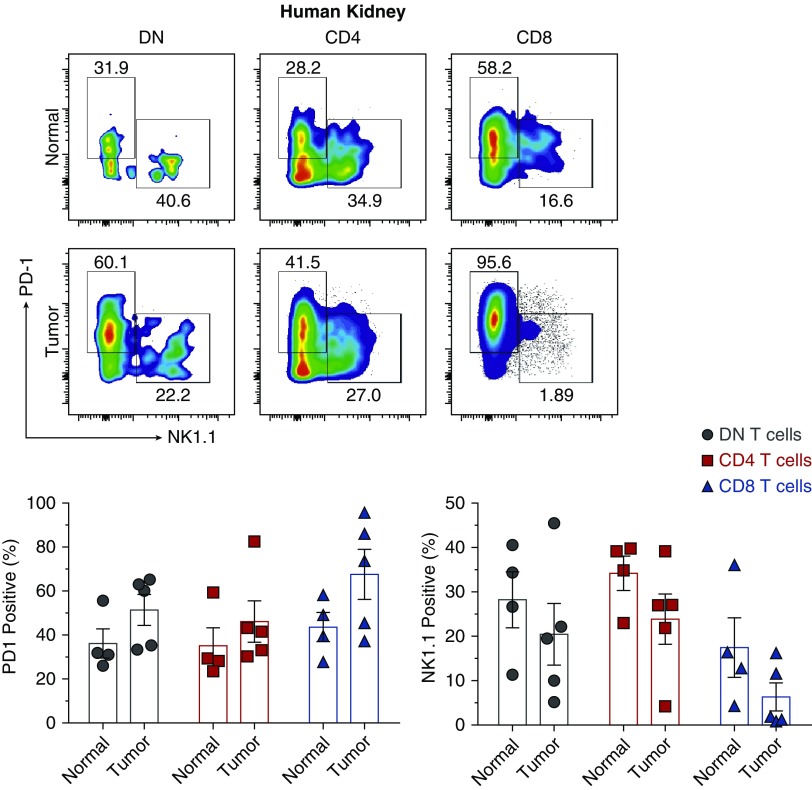
Detection of the NK1.1+ and PD-1+ DN T Cell Subsets in Human Kidneys
Our study identified two subsets of murine kidney DN T cells and used in vitro and in vivo experiments to elucidate homeostasis and regulation of kidney DN T cells in healthy and AKI conditions in mice. To test whether these data could also have translational potential in humans, we examined human kidneys for presence of the two distinct PD-1+ and NK1.1+ DN T cell subsets. We used the histologically “normal” part of kidneys removed for renal cell cancer, and examined the cancerous sample. Similar to our mouse findings, we detected the PD-1+ and NK1.1+ subsets of DN T cells in human kidneys (Figure 8). Their frequencies varied among the samples, and future studies should determine whether the heterogeneity is secondary to disease types or other factors such as age, sex, etc. In addition, almost all CD8 T cells in tumor kidney tissues expressed high levels of PD-1 compared with normal tissues, and PD-1–expressing CD4 T cells increased in cancerous tissues (Figure 8).
Figure 8.
Human kidneys have both PD-1+ and NK1.1+ DN T cell subsets similar to mouse kidneys. Lymphocytes were isolated from histologically normal and cancerous portions of nephrectomies from patients with renal cell carcinoma, CD45+ cells were gated, and frequency of PD-1+ and NK1.1+ cells within the CD4, CD8, and DN αβ T cell subsets were analyzed by flow cytometry. Dot plots show representative percentage of PD-1+ and NK1.1+ cells. Numbers in quadrants indicate percentages. Graphs show cumulative data. Data points (mean±SEM) are from at least four to five human samples.
Discussion
Our results demonstrate that DN T cells represent a unique population of kidney T cells that depend on nonclassic β2m molecules for their homeostasis and utilize MHC-independent mechanisms to respond to external stimuli. We found an important role for IL-2 provided by other T cells in DN T cell expansion, both in vitro and in vivo. In addition, we identified PD-1+ and NK1.1+ subsets, with expansion of the PD-1+ subset during ischemic AKI. We also demonstrated presence of both PD-1+ and NK1.1+ DN T cells in human kidney samples. These findings identify DN T cells as distinct from CD4 or CD8 T cells and argue against the prevalent view that DN T cells are CD8 or CD4 T cells that lost their coreceptors. If kidney DN T cells were derived from CD8 T cells, they should have been lost in β2m-deficient mice, and if derived from CD4 T cells, they should been lost in MHC II–deficient mice.
MHC molecules present foreign and self-antigens to various T cell types. CD4+ T cells recognize antigens in the context of MHC class II molecules and CD8+ T cells recognize antigens presented by classic MHC class 1a.25 Our results show that neither MHC 1a nor MHC II are required for homeostasis of kidney DN T cells. Instead, kidney DN T cells are regulated by nonclassic β2m-dependent MHC molecules. Indeed, the PD-1+ subset was able to potently respond to external stimuli caused by T cells or by ischemic AKI in the β2m KO mice. Interactions between PD-1 and PD-1 ligands on CD4 and CD8 T cells have been shown to affect their effector functions and differentiation.26,27 Additionally, PD-1–dependent interactions are necessary for Treg cells to mediate protection against IRI-induced AKI.28 Future experiments should determine whether PD-1–mediated mechanisms regulates homeostasis, trafficking/recruitment, and/or functions of DN T cell in the kidney, which is currently made particularly challenging by paucity of sorted kidney DN T cells. Furthermore, the steady-state proliferation of DN T cells and their ability to sense infused autologous T cells and their extremely rapid response to IRI suggest a role in the lymphoid stress-surveillance program29 in the kidney.
The ability of DN T cells to respond to the infusion of T cells or ischemic AKI are composed of at least two responses, as infused B cells caused upregulation of CD25 but not proliferation of DN T cells. This indicates that B cell presence was sensed by DN T cells, but a second stimulatory signal is needed for proliferation and not provided by B cells. However, the latter signal is provided by T cells, and it depends on IL-2 as shown by our in vitro experiments. DN T cells are regulatory cells and similar to FoxP3-expressing T regulatory cells require IL-2 for their activation and function.30 IL-2 is mainly produced by activated CD4 and CD8 T cells and consumed preferentially by T cells expressing CD25, the high-affinity IL-2Rα.31
The importance of the PD-1+ subset in kidney is currently unknown, but potentially very relevant given the marked success of immune checkpoint therapy directed toward PD-1+ for cancer immunotherapy.32,33 Thus, our findings set the stage for further exploration into the biology and precise functions of kidney DN T cells in the steady state, as well as therapeutic potential for AKI, malignancy, GN, transplant rejection, and other immune cell–mediated kidney diseases.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank National Institutes of Health (NIH) tetramer core facility for CD1d tetramer. We also acknowledge a generous research gift from Rogelio Miro of Metales, S.A., Panama.
M.S. designed and performed most of the experiments, interpreted results, and wrote the manuscript. S.N. provided advice on the project design and prepared the manuscript. S.A.L. provided advice on the project design, performed kidney ischemia-reperfusion model, and prepared the manuscript. J.G. performed experiments and prepared the manuscript. M.E.A. and P.P. provided human kidney tissues for the study and provided guidance on kidney cancer. H.R. supervised the project, was involved in project design and wrote the manuscript. A.R.A.H. supervised the project, advice on the project design, interpreted results, and wrote the manuscript.
This study is supported by NIH grant R01-DK104662.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2018080815/-/DCSupplemental.
Supplemental Figure 1. Gating strategy for identification of different T cell subsets.
Supplemental Figure 2. β2m deficiency significantly reduces relative and absolute number of thymic DN T cells, but not in lymph nodes.
Supplemental Figure 3. Lack of β2m does not affect CD44 and CD28 expression by kidney DN T cells.
Supplemental Figure 4. Kidney DN T cells express Fas/FasL and few express B220 and Thy1.2.
Supplemental Figure 5. β2m deficiency leads to loss of NK1.1+ DN T cells in the thymus but not in the lymph node.
Supplemental Figure 6. Restoration of kidney DN T cells by adoptively transferred CD8 T cells was not due to downregulation of CD8 coreceptors by donor T cells.
Supplemental Figure 7. Injection of β2m KO mice with CD4 T cells caused activation and expansion of kidney DN T cells, whereas injection of B cells caused activation without expansion.
Supplemental Figure 8. Expansion of DN T cells with CD8 or CD4 T cells from spleen during in vitro culture.
Supplemental Figure 9. Injection of CD8 T cells into β2m KO mice enhance expression of CD25 and IL-2 in kidney DN T cells.
References
- 1.Grinyó JM: Why is organ transplantation clinically important? Cold Spring Harb Perspect Med 3: a014985, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suárez-Fueyo A, Bradley SJ, Klatzmann D, Tsokos GC: T cells and autoimmune kidney disease. Nat Rev Nephrol 13: 329–343, 2017 [DOI] [PubMed] [Google Scholar]
- 3.Cojocaru M, Cojocaru IM, Silosi I, Vrabie CD: Kidney damage in autoimmune diseases. J Med Biochem 29: 61–65, 2010 [Google Scholar]
- 4.Rao J, Lu L, Zhai Y: T cells in organ ischemia reperfusion injury. Curr Opin Organ Transplant 19: 115–120, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascon DB, Ascon M, Satpute S, Lopez-Briones S, Racusen L, Colvin RB, et al.: Normal mouse kidneys contain activated and CD3+CD4- CD8- double-negative T lymphocytes with a distinct TCR repertoire. J Leukoc Biol 84: 1400–1409, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martina MN, Noel S, Saxena A, Bandapalle S, Majithia R, Jie C, et al.: Double-negative αβ T cells are early responders to AKI and are found in human kidney. J Am Soc Nephrol 27: 1113–1123, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruscher R, Kummer RL, Lee YJ, Jameson SC, Hogquist KA: CD8αα intraepithelial lymphocytes arise from two main thymic precursors. Nat Immunol 18: 771–779, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Acquisto F, Crompton T: CD3+CD4-CD8- (double negative) T cells: Saviours or villains of the immune response? Biochem Pharmacol 82: 333–340, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Kisielow P, Teh HS, Blüthmann H, von Boehmer H: Positive selection of antigen-specific T cells in thymus by restricting MHC molecules. Nature 335: 730–733, 1988 [DOI] [PubMed] [Google Scholar]
- 10.Schiefner A, Wilson IA: Presentation of lipid antigens by CD1 glycoproteins. Curr Pharm Des 15: 3311–3317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keller AN, Corbett AJ, Wubben JM, McCluskey J, Rossjohn J: MAIT cells and MR1-antigen recognition. Curr Opin Immunol 46: 66–74, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Rodgers JR, Cook RG: MHC class Ib molecules bridge innate and acquired immunity. Nat Rev Immunol 5: 459–471, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Martina MN, Bandapalle S, Rabb H, Hamad AR: Isolation of double negative αβ T cells from the kidney. J Vis Exp (87): 51192, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster B, Prussin C, Liu F, Whitmire JK, Whitton JL: Detection of intracellular cytokines by flow cytometry. Curr Protoc Immunol Chapter 6: Unit 6.24, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Trickett A, Kwan YL: T cell stimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods 275: 251–255, 2003 [DOI] [PubMed] [Google Scholar]
- 16.Morgan E, Varro R, Sepulveda H, Ember JA, Apgar J, Wilson J, et al.: Cytometric bead array: A multiplexed assay platform with applications in various areas of biology. Clin Immunol 110: 252–266, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Lin GH, Stone JC, Surh CD, Watts TH: In vivo accumulation of T cells in response to IL-2/anti-IL-2 mAb complexes is dependent in part on the TNF family ligand 4-1BBL. Immunol Cell Biol 90: 743–747, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabb H, Ramirez G, Saba SR, Reynolds D, Xu J, Flavell R, et al.: Renal ischemic-reperfusion injury in L-selectin-deficient mice. Am J Physiol 271: F408–F413, 1996 [DOI] [PubMed] [Google Scholar]
- 19.Vugmeyster Y, Glas R, Pérarnau B, Lemonnier FA, Eisen H, Ploegh H: Major histocompatibility complex (MHC) class I KbDb -/- deficient mice possess functional CD8+ T cells and natural killer cells. Proc Natl Acad Sci U S A 95: 12492–12497, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borland G, Ross JA, Guy K: Forms and functions of CD44. Immunology 93: 139–148, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baaten BJ, Li CR, Bradley LM: Multifaceted regulation of T cells by CD44. Commun Integr Biol 3: 508–512, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erard F, Wild MT, Garcia-Sanz JA, Le Gros G: Switch of CD8 T cells to noncytolytic CD8-CD4- cells that make TH2 cytokines and help B cells. Science 260: 1802–1805, 1993 [DOI] [PubMed] [Google Scholar]
- 23.Létourneau S, van Leeuwen EM, Krieg C, Martin C, Pantaleo G, Sprent J, et al.: IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proc Natl Acad Sci U S A 107: 2171–2176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasteiger G, Hemmers S, Bos PD, Sun JC, Rudensky AY: IL-2-dependent adaptive control of NK cell homeostasis. J Exp Med 210: 1179–1187, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viret C, Janeway CA Jr.: MHC and T cell development. Rev Immunogenet 1: 91–104, 1999 [PubMed] [Google Scholar]
- 26.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, et al.: PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol 32: 634–643, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Ahn E, Araki K, Hashimoto M, Li W, Riley JL, Cheung J, et al.: Role of PD-1 during effector CD8 T cell differentiation. Proc Natl Acad Sci U S A 115: 4749–4754, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jaworska K, Ratajczak J, Huang L, Whalen K, Yang M, Stevens BK, et al.: Both PD-1 ligands protect the kidney from ischemia reperfusion injury. J Immunol 194: 325–333, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aptsiauri N, Cabrera T, Garcia-Lora A, Lopez-Nevot MA, Ruiz-Cabello F, Garrido F: MHC class I antigens and immune surveillance in transformed cells. Int Rev Cytol 256: 139–189, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Juvet SC, Zhang L: Double negative regulatory T cells in transplantation and autoimmunity: Recent progress and future directions. J Mol Cell Biol 4: 48–58, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smigiel KS, Srivastava S, Stolley JM, Campbell DJ: Regulatory T-cell homeostasis: Steady-state maintenance and modulation during inflammation. Immunol Rev 259: 40–59, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flemming A: Cancer: PD1 makes waves in anticancer immunotherapy. Nat Rev Drug Discov 11: 601, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al.: PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: Mechanism, combinations, and clinical outcome. Front Pharmacol 8: 561, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.