Figure 9.
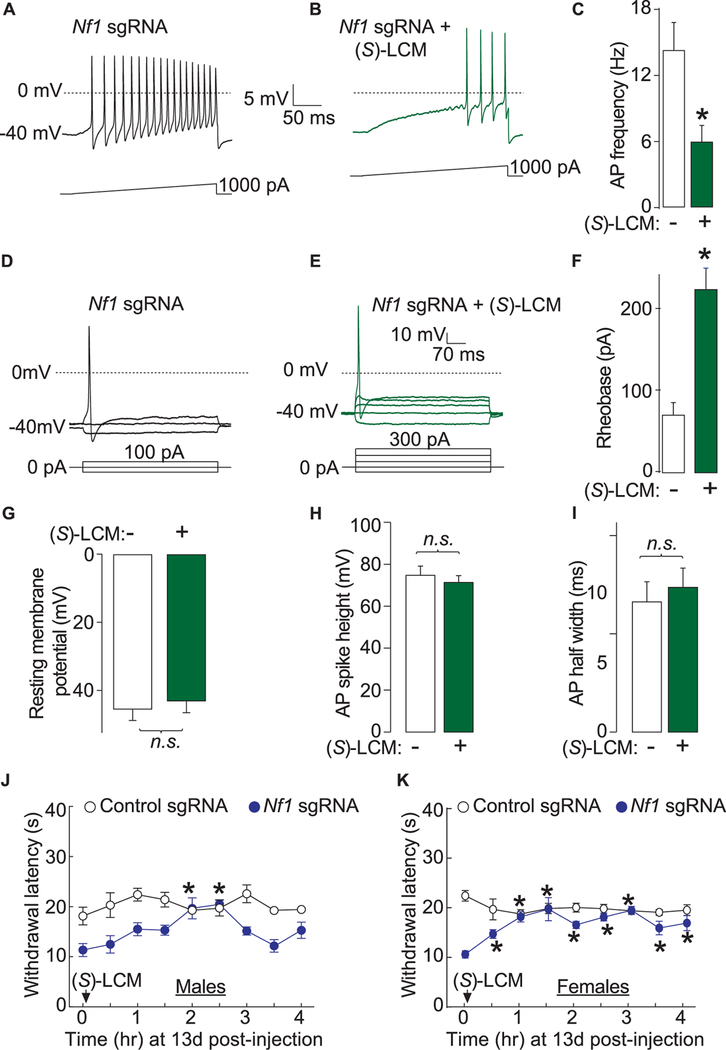
(S)-LCM reverses excitability and thermal hyperalgesia after CRISPR/Cas9 Nf1 editing in rats. Representative recordings in response to a step of depolarizing current evoked action potentials (APs) in sensory neurons transfected with neurons transfected by Nf1 sgRNA containing plasmid and treated with either DMSO 0.04% (A) or 10 μM (S)-LCM (B). (C) Summary of the number of APs in the indicated conditions (n = 11 cells for Nf1 sgRNA 1 DMSO 0.04% and n = 12 cells for Nf1 sgRNA +10 μM (S)-LCM). Representative recordings in response to various steps of depolarizing current to measure rheobase in sensory neurons transfected with neurons transfected by Nf1 sgRNA containing plasmid and treated with either DMSO 0.04% (D) or 10 μM (S)-LCM (E). Rheobase is the current required for eliciting the first AP. (F) Summary of the measured rheobase in indicated conditions (n = 11 each). Summary of the resting membrane potential (in millivolts, mV) (G), AP spike height (in mV) (H), and AP half width (in milliseconds, ms) (I) in the indicated conditions. Asterisks indicate significance compared with control sgRNA transfected cells (*P < 0.05, Student’s t test). n.s., not-significant; P > 0.05; unpaired Student’s t test. At least 11 to 14 cells were recorded from for the parameters shown in (G-I). (G) Thermal hyperalgesia (paw withdrawal latency) of male (J) and female (K) rats, 13 days after in vivo transfection with control or Nf1 sgRNA containing plasmids. Male rats (n = 6–8) were orally administered 30 mg/kg of (S)-LCM and their paw withdrawal latency was followed for 4 hours. *P < 0.05 vs control sgRNA, Student’s t test. Error bars represent mean ± SEM.
