Abstract
The present aging rate in Japan of some 28% will continue to increase along with the advancing age of elderly persons. Therefore, the demand for care will also increase. Approximately 25% of the need for nursing-care defined by the Japanese long-term care insurance system is associated with disorders or deterioration of locomotive organs. Therefore, the prevention and treatment of diseases in the locomotor system and maintenance of motor function are important for extended healthy life span and to decrease the demand for long-term care. Based on this background, the Japanese Orthopaedic Association (JOA) proposed the concept of locomotive syndrome (LS) in 2007, which is defined as reduced mobility due to impaired locomotive organs. Changes in locomotion must be noticed early to ensure the timely implementation of appropriate checks and measures of locomotion can uncover risk of acquiring LS. The acquisition of an exercise habit, appropriate nutrition, being active and evaluating and treating locomotion-related diseases are important to delay or avoid LS. The JOA recommends locomotion training consisting of four exercises to prevent and improve LS. Countermeasures against LS should become a meaningful precedent not only for Japan, but for other countries with rapidly aging populations.
Keywords: Locomotive syndrome, Loco-check, Locomotion training
1. Background of the locomotive syndrome concept
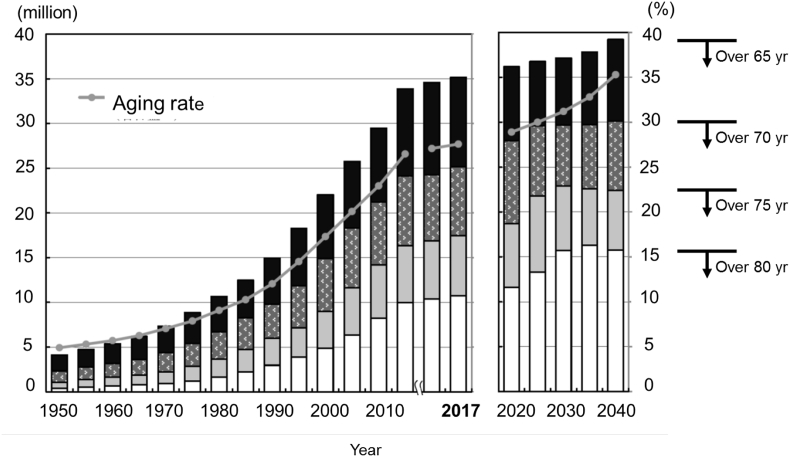
The aging rate in Japan exceeded 21% in 2007, resulting in Japan being the earliest super-aged society in the world [1]. The number of elderly persons living in Japan exceeded 35 million during 2017 and the aging rate almost reached 28%. Estimates indicate that the aging rate in Japan will increase to 30% in 2025, and 35% in 2040 (Fig. 1) [2].
Fig. 1.
Population aged >65 years (column) and aging rates (line) in Japan between 1950 and 2040. Data up to 2017 and after 2020 are from statistics prepared by Japanese Ministry of Health, Labor and Welfare, Japan and estimates prepared by National Institute of Population and Social Security Research, respectively. The graph is quoted from Japan Ministry of Internal Affairs and Communication sources.
Though the population in Japan has declined since 2015, people aged over 75 and 90 years have remarkably increased and this trend will continue [2]. Therefore, the aging of elderly persons has resulted in increased demand for care. This problem is likely to affect other countries in the near future.
The long-term care insurance system in Japan defines recipients of long-term nursing care according to standards of severity and provides care services according to these standards at home or in facilities. During 2017, 6.2 million elderly persons were deemed eligible for long-term care and its annual cost amounted to over 10 trillion yen (approximately USD $90 billion) by Japanese governmental statistics. According to this system, the following conditions are eligible to receive nursing: dementia (18.0%), cerebrovascular diseases (16.6%), frailty (13.3%), joint diseases (12.1%), falls and fractures (10.2%), and spinal cord injury (2.3%) [3]. Consequently, disorders of the locomotive system account for about 25% of the national need for long-term care. Therefore, maintaining independence among elderly persons by preventing a decline in motor function and preventing/improving dysfunctions of locomotive organs are indispensable for the long-term quality of life (QoL).
From the viewpoint of promoting locomotive health among elderly persons, the JOA proposed the concept of locomotive syndrome (LS) in 2007. LS is defined as a state of degraded mobility due to impaired locomotive organs, which elevates risk of disability [4,5]. Disorders of the locomotive system include deteriorated motor functions and musculoskeletal pathologies, including osteoporosis, fractures, osteoarthritis of the knee and hip joints, degenerative spondylosis, spinal canal stenosis and sarcopenia. Some of these disorders may not directly affect the mobility function, especially in early stages. Also, osteoporosis without fragility fracture has no influence on the mobility. However, because we aimed for the prevention of disability or care-need conditions, we listed these diseases as associated with LS. Motor functions deteriorate with age and musculoskeletal diseases are more likely to occur and progress among elderly persons. Progression of LS among elderly persons results in deteriorating mobility that eventually results in a demand for care. Thus, preventing LS is crucial to an extended, good QoL in a super-aged society.
In addition to aging, diseases of organs and genetic factors also influence LS. Variable factors such as a lack of habitual exercise, sedentary lifestyles, and inadequate nutritional intake also cause progressive LS. Improvement of these factors and the prevention and treatment of musculoskeletal diseases comprise the major strategy against LS. Furthermore, it is important to prevail the concept and importance of LS, along with the simple and efficient evaluation method and measures for it to the general public.
In terms of decline of physical functions in aged people, the concepts of sarcopenia and frailty are well-known and the criteria of those are established. Sarcopenia is defined as the condition of decreased muscle mass of extremities [6], accompanied with decreased grip power or gait speed [7,8]. In the criteria of sarcopenia, muscle mass reduction is essential for the diagnosis, so there is a possibility of missing mobility degradation without decreasing muscle mass, such as dynapenia. So, we proposed the concept of LS that specifically relates mobility itself. Frailty means a state in which morbidity, disability, and death are strongly predicted [9]. Frailty includes not only physical factors but also mental and social elements. So, it is a wide and inclusive concept with criteria capturing a wide range of factors. We, therefore, needed more specific concept and criteria for impaired mobility like LS.
2. Characteristics of LS
Musculoskeletal diseases associated with LS are highly prevalent among elderly persons, many of whom have several concurrent diseases. Yoshimura et al. [10] estimated from epidemiologic surveys of 3000 Japanese residents aged >40 years living in mountainous areas, fishing villages and urban areas that 37.9, 25.3, and 12.8 million people in the nation have degenerative lumbar spinal spondylosis (diagnosed by Kellgren-Lawrence X-ray criteria), knee osteoarthritis and osteoporosis (diagnosed from bone mineral density of the lumbar spine and femoral neck), respectively. The estimated number of elderly persons affected by at least one of these three conditions was 47 million and 24.7 million have combinations of them.
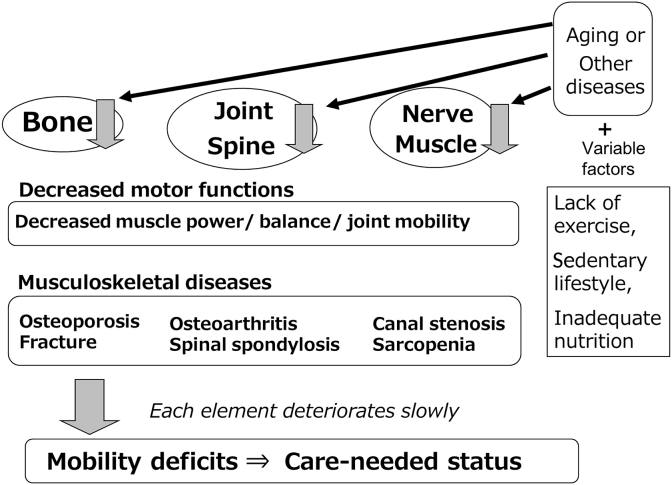
Fig. 2 shows the concepts and progression of LS. Musculoskeletal diseases progress slowly with age and are interrelated. The locomotive system consists of muscles, joints, cartilage, bones including the spine, intervertebral discs and nerves. All organs and tissues inevitably weaken with age. In particular, muscles and bones weaken in the absence of habitual exercise and progress to sarcopenia and osteoporosis. Decreased muscle strength can cause or advance arthropathy [11,12]. Pain and restricted movement due to arthropathy and spondylosis also may cause muscle and bone weakening. Thus, locomotive organs slowly deteriorate in an interrelated fashion, which will result in dysfunctional mobility, dependence, a need for long-term care, and a bedridden future.
Fig. 2.
Concepts and progression of locomotive syndrome. Locomotive syndrome is defined as declining mobility resulting from locomotive system disorders including decreased motor functions and musculoskeletal diseases that cause mobility deficits and demand for care. Besides aging and diseases of other organs or tissues, various factors such as lack of habitual exercise, sedentary lifestyle and inadequate nutrition accelerate progression of locomotive syndrome.
These facts stress the importance of identifying LS early and preventing its progression.
3. Risk assessment and judging tools of LS
The JOA created the self-administered locomotion check (Loco-check) for LS and the short test battery for locomotive syndrome (STBLS) to assess risk of LS. The Loco-check consists of a simple 7-item questionnaire that can be implemented alone. The STBLS comprises Stand-Up and Two-Step tests of motor function and a 25-item questionnaire, named 25-question Geriatric Locomotive Function Scale (GLFS-25).
3.1. Loco-check
Since the prevalence of LS is high and there are few symptoms in the early stage, we need a measure to know the decline of motor function at an early stage. Therefore, we created a Loco-check, a simple self-check method for scenting the risk of LS. Loco-check consists of the following items. Risk of LS is defined as having at least one of these items [13].
-
1
Unable to put on a sock while standing on one leg
-
2
Frequent trips or slips around the house
-
3
Need to hold a handrail when climbing stairs
-
4
Difficulty doing moderately heavy housework
-
5
Difficulty carrying home 2 kg of shopping
-
6
Unable to walk for 15-min nonstop
-
7
Unable to cross a street before the light turns red
Because Loco-check questions general everyday behavior, anyone can easily answer it. Each item is selected from some questionnaires such as GLFS-25 [14], a fall risk index [15], and the Kihon Checklist, which screens the degree of daily autonomy and motor function disorders as well as frailty [16] of elderly persons used by the Japanese government to determine need for care.
Decreased motor functions are indicated when LS is screened by Loco-check. Among 219 ambulatory community-dwellers (average age, 75.9 years; female, n = 188; male, n = 29), average results were worse for a group that was positive (39.7% of the total), than negative in the Loco-check in terms of amount of one-leg standing test, gait speed, timed up-and-go test, knee extension strength, and flexion strength of the foot toes [17].
Loco-check positiveness indicated radiographic knee osteoarthritis, loss of skeletal muscle mass, balancing and flexibility [18]. The number of those who have the history of falls is larger according to the presence or absence of the applicable item in the Loco-check and the number of items [19]. In addition, the utility value was low for the EQ-5D (EuroQOL-5 dimensions) in a Loco-check positive group [20]. Therefore, the Loco-check identifies people at risk for ambulatory dysfunction or LS.
3.2. Short test battery for locomotive syndrome
STBLS is a set of assessment tools for screening LS comprising the Stand-Up test to evaluate the muscle power of lower extremities, the Two-Step test to evaluate length of strides that relates gait speed, and the GLFS-25, a questionnaire that assesses symptoms and functions of the locomotor system [21]. It is supposed to be utilized by medical professionals or researchers, while Loco-check is a self-checking tool. STBLS determines the presence and degree of LS, while Loco-check suggests the elevated risk of LS.
Numerous evaluation tools for frailty have been reported [22]. Among them, the Cardiovascular Health Study (CHS) standard recommended by Fried et al. [9], the advocate of frail concept, is most frequently used. In Japan, the J-CHS standard that matches CHS standards to japan is popularly used. Both of them consist of 5 constituent concepts, of which the evaluation of motor function is gait speed and grip strength.
Gait speed is a most popular and established measurement to evaluate mobility, so the criteria of both sarcopenia and frailty adopt it [[7], [8], [9]]. In our daily lives, however, we move in a vertical direction, as well as in a horizontal direction. In other words, we stand up and walk for moving ourselves. So, evaluation methods for both directions of mobility are necessary. The Stand-Up test examines the ability for a vertical movement, and the Two-Step test examines that for a horizontal movement.
Grip strength is also a good index to correlate with muscular strength of the whole body, but grip strength is not expected to improve even by exercising lower limbs and trunk which improves mobility function.
Therefore, it is necessary for the general public to easily measure with a small space and to improve by intervention improving mobility. That is a reason why a new method of evaluating functions directly relating mobility.
Moreover, symptoms and functions of musculoskeletal organs are important factors for evaluating LS, because such factors may lead immobility. GLFS-25 screens symptoms and functions of musculoskeletal organs. So, JOA proposed a set of those three measurements for LS.
So far, records of these tests have shown an age-dependent decline in locomotor function among 777 Japanese people, as well as the test-retest reliability of the Stand-Up test, the Two-Step test [21].
Table 1 summarizes the STBLS stage criteria. When a single test in STBLS results in LS stage 1, LS is considered to have started and measures to establish or improve exercise habits and appropriate nutrition are recommended. Stage 2 indicates progressive LS with possible musculoskeletal degeneration that requires clinical consultation. Individuals who are judged as being free of LS are also encouraged to implement preventive measures.
Table 1.
Judging criteria of locomotive syndrome (LS) stages 1 and 2 for short test battery for locomotive syndrome.
| Stage | Stand-Up test | Two-Step test | GLFS-25 |
|---|---|---|---|
| 1 | Fail at one-leg/40 cm | <1.3 | ≥7 points |
| 2 | Fail at both-legs/20 cm | <1.1 | ≥16 points |
LS stage 1 indicates the onset of LS. Consider habitual exercises and nutritional improvement. LS stage 2 indicates advanced LS. Consider habitual exercises and nutritional improvement, as well as see a doctor to check if you have musculoskeletal diseases.
GLFS-25, 25-question Geriatric Locomotive Function Scale.
STBLS can be used to evaluate mobility functions from young adults to the elderly. It appears to be useful from preventive medicine to evaluation of treatment for musculoskeletal diseases. It is spreading in areas such as health checkups by government and on a commercial basis in Japan. On the other hand, it is also used to evaluate the outcomes of treatment or surgeries for musculoskeletal diseases, such as total arthroplasty [23].
3.2.1. Stand-Up test
The Stand-Up test measures the muscle power of the lower extremities (Fig. 3) [24]. Individuals are required to stand up from being seated on 10-, 20-, 30-, and 40-cm-high stools using one or both legs. The test usually starts by standing up from a 40-cm-high stool on one leg. The trial is judged as successful if the person can stand up and hold the position for >3 seconds. Having passed that test, the same person will attempt to stand up on one leg, and if unable to do so, on both legs, from a 30-cm-high stool. The Stand-Up test was shown to be associated with, bone density and gait speed [25]. Being unable to stand up from a 40-cm-high stool is judged as LS stage 1. Being unable to stand up from 20-cm stool with both legs is judged as LS stage 2.
Fig. 3.
Two-Step test. This test measures stride length to assess walking ability, muscular strength, balance, and lower limb flexibility. The Two-Step value, double strides divided body height and correlates with gait speed.
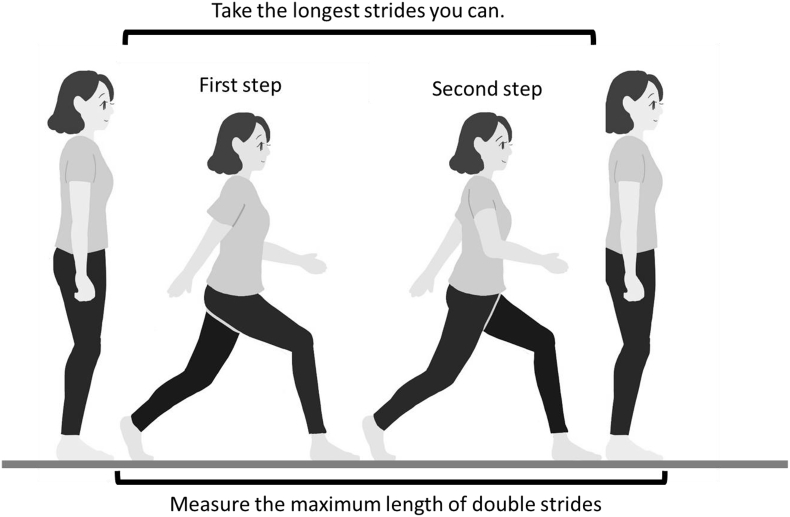
3.2.2. Two-Step test
The Two-Step test screens walking ability (Fig. 4) [26]. Individuals stand on a starting line and take 2 steps forward at maximum stride while trying to maintain balance and not fall, and then stop on both feet. The distance travelled during the 2 steps is measured and divided by the height of the individual to obtain the Two-Step value. The test is implemented twice, and the best result is taken. The Two-Step value closely correlates walking speed. Values < 1.3 and < 1.3 are rated as LS stages 1 and 2, respectively.
Fig. 4.
Stand-Up test. This test assesses leg strength by standing up on one or both legs from seats of varying height.
3.2.3. Geriatric Locomotive Function Scale of 25 questions
This self-administered questionnaire evaluates pain and numbness, motor dysfunction and mobility in the day-to-day lives of middle-aged and elderly populations. The Geriatric Locomotive Function Scale of 25 questions (GLFS-25) score correlates with mobility defined by clinical experts as being robust, requiring support, or requiring care [10]. Each question has five alternatives scored from 0 to 4 from better to worse, and total scores between 0 and 100 are calculated for 25 questions. In the studies for elderly people, GLFS-25 was associated with lower gait speed [27], speed of ultrasound in a calcaneal bone [28], and fall risk [29]. Scores of ≥7 and ≥ 16 are rated as LS stages 1 and 2, respectively.
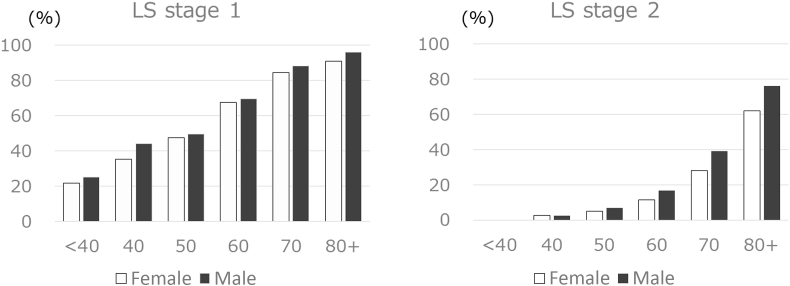
The ROAD (Research on Osteoarthritis Against Disability) study investigated the age-specific rate of each stage LS stage in a large regional residential cohort in Japan that consisted of 3000 community-dwelling people aged 40s–80s (Fig. 5) [30]. The prevalence of LS stage 1 was 69.8% of the total (males, 68.4%; females, 70.5%), and that of LS-stage 2 was 25.1% of the total (males, 22.7%; females, 26.3%). That study also found that the prevalence of LS stages 1 and 2 increases with age, reaching 50% of those aged in their 70s.
Fig. 5.
Prevalence of locomotive syndrome (LS) stages 1 and 2. A large cohort study that consisted of 3000 community-dwelling people aged 40s–80s investigated the age-specific rate of each stage LS stage in a large regional residential cohort in Japan.
4. Prevention of the onset and progression of LS
Many investigators have found that having an exercise habit or undergoing exercise intervention can maintain or improve motor functions, musculoskeletal diseases such as osteoporosis [[31], [32], [33], [34], [35]], osteoarthritis [36], and sarcopenia among geriatric persons. Quadriceps training [37] decreases symptoms of knee osteoarthritis. Strengthening back muscles improves low back pain [38] and exercise intervention can improve risk of vertebral fracture [39], balance [40], prevent falls [41]. Appropriate nutritional intake is also important to maintain and improve motor function among elderly persons [[42], [43], [44]]. Therefore, to acquire an exercise habit and maintain appropriate nutritional intake are important to prevent and improve LS.
In terms of exercises for LS, safe, simple, effective multifactorial exercises including balance and muscle strengthening are recommended for life. The JOA therefore proposed sets of exercises for LS called locomotion training (LT) comprising standing on one leg with the eyes open, squats, heel raises and front lunges. The objective of LT is mainly strengthening the muscle power of the lower extremities and improving balance, which is indispensable for ambulation and basic activities of daily living. These exercises have been proven effective in a survey of intervention for middle-aged and elderly persons in Japan [17,45].
The following describes each LT exercise recommended by JOA.
4.1. One-leg standing with eyes open
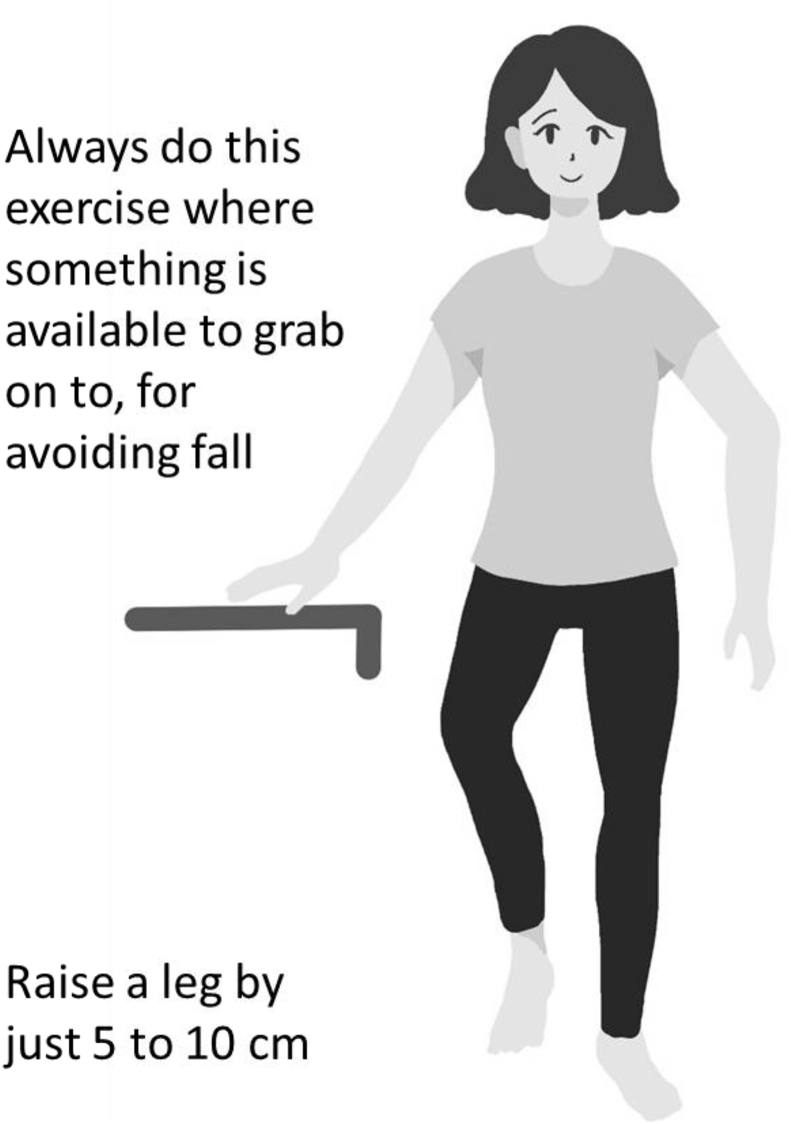
Stability while standing on one leg is important for walking, climbing stairs and avoiding fall risk. Sakamoto et al. [40] named this exercise “dynamic flamingo therapy” and implemented intervention trials in which elderly persons implemented this exercise for 6 months. The results showed that the incidence of falls was reduced by about 33% in the intervention group compared with a control group.
This exercise simply requires raising one foot by 5–10 cm and standing on the other with the eyes open (Fig. 6) for 1 min per foot three times a day.
Fig. 6.
Standing on one leg with eyes open. This exercise mainly improves balance. Three repetitions per day of 1 min per leg are recommended.
If unstable while standing on one leg, the exercise can be implemented while lightly touching a support with the fingers. Touching with a single finger can facilitate balance. Risk of falls is important for elderly persons and this exercise should be implemented near a surface that can easily be touched such as a wall or grasped such as a desk, chair or handrail.
4.2. Squats
Squats efficiently increase muscle strength and improve that of the entire lower limbs (Fig. 7); thus, they are often included in sport training and exercises for elderly persons. Electromyographic evidence has indicated that squats cause contraction of the gluteus maximus, gluteus medius, quadriceps femoris, hamstrings and tibialis anterior muscles [46,47].
Fig. 7.
Squats. This simple, safe exercise strengthens lower extremity muscles. Posture with knees bent behind toes is important for correct squat technique. One squat should take 5 seconds to lower into position and 5 seconds to return to standing upright. One-to-three sets of 5–15 repetitions per day are recommended.
Squats require bending the knee while pulling the buttocks to the back so that the knee does not extend over the toes. Both the quadriceps muscle that extends the knee and the gluteus maximus are necessary for hip joint extension when squatting in this manner; the hamstrings involved in knee flexion also contract so the training effect is high and inclusive. The upper body may be inclined forwards, and/or the hands may be stretched forwards to maintain balance. Approximately 5 seconds each are required to squat and to stand up. The aim is to achieve three sets per day of 5–10 repetitions. Slowly standing up and sitting down on a chair has the same effects as squats when standing is unstable or walking is impossible.
4.3. Heel raises
The heels are raised and lowered while standing on the balls of the feet to reinforce the triceps muscles of the lower leg (Fig. 8). This exercise develops the muscle strength of plantar flexion [48] that corelates gait speed [49] and may also improve toe flexor strength that corelates fall risk [50].
Fig. 8.
Heel raises. This exercise strengthens gastrocnemius and soleus muscles (triceps) of lower extremities. Two-to-three sets of 10–20 raises per day are recommended.
4.4. Front lunges
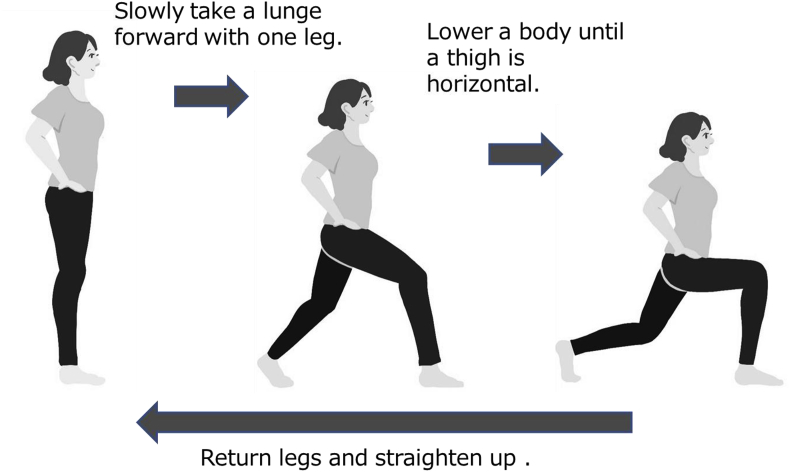
Front lunges effectively activate muscle powers of the lower extremities [51]. Place each hand on the iliac spines, step forward with one foot in line with the back foot, slowly lower the upper body by flexing the hip and knee joints, stand up, and return the foot to the initial position (Fig. 9). This series of actions uses most muscles of the lower limbs. However, care should be taken to ensure that elderly persons do not implement this exercise alone to avoid falls.
Fig. 9.
Front lunges. This exercise improves flexibility, balance, and muscular strength of lower limbs. Two-to-three sets of 5–10 lunges per day are recommended.
4.5. Usability and effects on physical functions of LT
LT is a set of low-to medium-intensity exercises with high safety, so it appears to be applicable to a wide range of people from young adults to the elderly. Also, LT can be conducted from a robust person to a frail or sick person if one can stand up by oneself. However, effects of intervention with LT have been reported so far just on the healthy old persons as follows.
When 6-month intervention by one-leg standing and squats was performed on the elderly who had one or more items in loco-check, the one-leg standing time and functional reach test improved significantly and the number of items of loco-check decreased significantly [52]. Intervention on 217 elderly subjects for 2 months by one-leg standing, squats, and heel raises resulted in the significant improvement of one-leg standing time, functional reach test, gait speed, 3-m Timed Up and Go test, and toe-grip strength [53]. When the 28 elderly subjects corresponding one or more items of loco-check continued LT and walking for 3 months, one-leg standing time and knee flexion strength, and also the loco-check corresponding number of items and the fears of falls significantly decreased [54]. The home-exercise intervention by LT realized a high continuation rate of about 90% in 3 months with a method called, “locomo-call,” characterized by 1 or 2 phone calls per week to encourage the participants to continue LT exercises. As a result, one-leg standing time increased significantly [55].
Thus, LT appears to improve physical functions of the healthy elderly people. However, there is no randomized-control studies to see the effects of LT or studies that evaluate the middle- or long-term effects of LT on prevention of the onset or progression of LS.
As well as exercises, nutritional interventions are proved to be important aspects. There are several studies that showed the additive effects of supplementation with protein [56,57], amino acids [58] to exercise intervention.
5. Current status and issues associated with LS in Japan
All of average life-span, aging rates, the number of persons requiring nursing care, medical expenses, and nursing care expenditures are continuously increasing in Japan. Japanese government statistics show that disorders of the locomotive organs are causing high expenses in both medical care and nursing care [4]. From this viewpoint, LS prevention is regarded as a national priority in the country.
The JOA established the Locomo-Challenge Council in 2010 to further prevail the concept of LS and measures for it. The council distributes LS pamphlets in Japanese and English at booths associated with numerous scientific meetings, collaborating companies and local communities. The JOA presented an abbreviation “Locomo” for LS in domestic to prevail a sense of intimacy to LS among public people. The Japan Health, Labor and Welfare Ministry mounted a campaign to promote health in 2012, with the aim of raising awareness of LS from 17.3% to 80% by 2022. Intermittent results have shown that awareness of “Locomo” increased from 17.3% in 2012 to 48.1% in 2018.
Many local governments in Japan are offering health education lectures about LS. A survey of 1740 local governments conducted by the Japan Osteoporosis Foundation in 2014 showed that 64.2% of them had implemented health education courses about LS for local residents [59]. Collaborative efforts between local governments and academia are also being implemented in several prefectures such as Chiba, Saitama, and Miyazaki in Japan.
The concepts of frailty, sarcopenia and LS are important for a healthy, extended life span and preventing the demand for long-term care is also vital in Japan where aging continues to evolve. Comparisons of judgment criteria indicate that LS will probably be detected earlier than frailty [60,61] and sarcopenia [8]. Therefore, to detect LS in an early stage using the GLFS is a simple and efficient way to prevent frailty and sarcopenia.
The aging of society in any country will result in significant problems like those currently in Japan. Thus, LS countermeasures getting started and advanced in Japan are likely to set a precedent that will become meaningful for many countries in the future.
Conflicts of interest
No potential conflict of interest relevant to this article was reported.
Footnotes
Peer review under responsibility of The Korean Society of Osteoporosis.
References
- 1.White paper on the aging society (Summary) Cabinet Office, Government of Japan; 2008. The state of the aging population.http://www8.cao.go.jp/kourei/whitepaper/w-2008/zenbun/20index.html [Internet] [cited 2018 Sep 18] [Google Scholar]
- 2.National Institute of Population and Social Security Research . National Institute of Population and Social Security Research; Tokyo (Japan): 2017 Jul. Population projections for Japan: 2016-2065. Population Research Series No.336.http://www.ipss.go.jp/pp-zenkoku/j/zenkoku2017/pp29_ReportALL.pdf [Internet] [cited 2018 Sep 18] [Google Scholar]
- 3.Ministry of Health, Labor and Welfare . Ministry of Health, Labor and Welfare; Tokyo (Japan): 2016. The outline of the results of National livelihood survey (2016)http://www.mhlw.go.jp/toukei/saikin/hw/k-tyosa/k-tyosa16/dl/05.pdf [Internet] [cited 2018 Sep 18] [Google Scholar]
- 4.Nakamura K. A "super-aged" society and the "locomotive syndrome". J Orthop Sci. 2008;13:1–2. doi: 10.1007/s00776-007-1202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura K. The concept and treatment of locomotive syndrome: its acceptance and spread in Japan. J Orthop Sci. 2011;16:489–491. doi: 10.1007/s00776-011-0108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg I.H. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990S–991S. doi: 10.1093/jn/127.5.990S. [DOI] [PubMed] [Google Scholar]
- 7.Cruz-Jentoft A.J., Baeyens J.P., Bauer J.M., Boirie Y., Cederholm T., Landi F. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L.K., Liu L.K., Woo J., Assantachai P., Auyeung T.W., Bahyah K.S. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 9.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 10.Yoshimura N., Muraki S., Oka H., Mabuchi A., En-Yo Y., Yoshida M. Prevalence of knee osteoarthritis, lumbar spondylosis, and osteoporosis in Japanese men and women: the research on osteoarthritis/osteoporosis against disability study. J Bone Miner Metabol. 2009;27:620–628. doi: 10.1007/s00774-009-0080-8. [DOI] [PubMed] [Google Scholar]
- 11.Slemenda C., Heilman D.K., Brandt K.D., Katz B.P., Mazzuca S.A., Braunstein E.M. Reduced quadriceps strength relative to body weight: a risk factor for knee osteoarthritis in women? Arthritis Rheum. 1998;41:1951–1959. doi: 10.1002/1529-0131(199811)41:11<1951::AID-ART9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Baker K.R., Xu L., Zhang Y., Nevitt M., Niu J., Aliabadi P. Quadriceps weakness and its relationship to tibiofemoral and patellofemoral knee osteoarthritis in Chinese: the Beijing osteoarthritis study. Arthritis Rheum. 2004;50:1815–1821. doi: 10.1002/art.20261. [DOI] [PubMed] [Google Scholar]
- 13.Japanese Orthopaedic Association . Japanese Orthopaedic Association; Tokyo (Japan): 2016. Locomotive syndrome pamphlet 2015.https://locomo-joa.jp/en/index.pdf [Internet] [cited 2018 Sep 18] [Google Scholar]
- 14.Seichi A., Hoshino Y., Doi T., Akai M., Tobimatsu Y., Iwaya T. Development of a screening tool for risk of locomotive syndrome in the elderly: the 25-question Geriatric Locomotive Function Scale. J Orthop Sci. 2012;17:163–172. doi: 10.1007/s00776-011-0193-5. [DOI] [PubMed] [Google Scholar]
- 15.Toba K., Okochi J., Takahashi T., Matsubayashi K., Nishinaga M., Yamada S. Development of a portable fall risk index for elderly people living in the community. Nihon Ronen Igakkai Zasshi. 2005;42:346–352. doi: 10.3143/geriatrics.42.346. [DOI] [PubMed] [Google Scholar]
- 16.Satake S., Senda K., Hong Y.J., Miura H., Endo H., Sakurai T. Validity of the Kihon Checklist for assessing frailty status. Geriatr Gerontol Int. 2016;16:709–715. doi: 10.1111/ggi.12543. [DOI] [PubMed] [Google Scholar]
- 17.Ishibashi H., Fujita H. The effect of locomotion training on mobility function in the elderly female. Osteoporos Jpn. 2011;19:391–397. [Google Scholar]
- 18.Sasaki E., Ishibashi Y., Tsuda E., Ono A., Yamamoto Y., Inoue R. Evaluation of locomotive disability using loco-check: a cross-sectional study in the Japanese general population. J Orthop Sci. 2013;18:121–129. doi: 10.1007/s00776-012-0329-2. [DOI] [PubMed] [Google Scholar]
- 19.Akahane M., Maeyashiki A., Yoshihara S., Tanaka Y., Imamura T. Relationship between difficulties in daily activities and falling: loco-check as a self-assessment of fall risk. Interact J Med Res. 2016;5:e20. doi: 10.2196/ijmr.5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iizuka Y., Iizuka H., Mieda T., Tajika T., Yamamoto A., Takagishi K. Association between "loco-check" and EuroQol, a comprehensive instrument for assessing health-related quality of life: a study of the Japanese general population. J Orthop Sci. 2014;19:786–791. doi: 10.1007/s00776-014-0602-7. [DOI] [PubMed] [Google Scholar]
- 21.Ogata T., Muranaga S., Ishibashi H., Ohe T., Izumida R., Yoshimura N. Development of a screening program to assess motor function in the adult population: a cross-sectional observational study. J Orthop Sci. 2015;20:888–895. doi: 10.1007/s00776-015-0737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dent E., Kowal P., Hoogendijk E.O. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3–10. doi: 10.1016/j.ejim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 23.Uchida O., Sogo T., Takasago T., Iwase J., Higuchi T., Aoyama N. Effects of total hip arthroplasty on locomotive syndrome. Hip Joint. 2016;46:577–578. [Google Scholar]
- 24.Muranaga S., Hirano K. Development of a convenient way to predict ability to walk, using a two-step test. J Showa Med Assoc. 2003;63:301–308. [Google Scholar]
- 25.Ohsawa T., Shiozawa H., Saito K., Tajika T., Yamamoto A., Iizuka Y. Relation between the stand-up test and gait speed, knee osteoarthritis, and osteoporosis using calcaneal quantitative ultrasound - cross-sectional study. J Orthop Sci. 2016;21:74–78. doi: 10.1016/j.jos.2015.10.016. [DOI] [PubMed] [Google Scholar]
- 26.Muranaga S. Evaluation of the muscular strength of the lower extremities using the standing movement and clinical application. J Showa Med Assoc. 2001;61:362–367. [Google Scholar]
- 27.Nakamura M., Hashizume H., Oka H., Okada M., Takakura R., Hisari A. Physical performance measures associated with locomotive syndrome in middle-aged and older Japanese women. J Geriatr Phys Ther. 2015;38:202–207. doi: 10.1519/JPT.0000000000000033. [DOI] [PubMed] [Google Scholar]
- 28.Iizuka Y., Iizuka H., Mieda T., Tajika T., Yamamoto A., Takagishi K. Population-based study of the association of osteoporosis and chronic musculoskeletal pain and locomotive syndrome: the Katashina study. J Orthop Sci. 2015;20:1085–1089. doi: 10.1007/s00776-015-0774-9. [DOI] [PubMed] [Google Scholar]
- 29.Kimura A., Takeshita K., Inoue H., Seichi A., Kawasaki Y., Yoshii T. The 25-question Geriatric Locomotive Function Scale predicts the risk of recurrent falls in postoperative patients with cervical myelopathy. J Orthop Sci. 2018;23:185–189. doi: 10.1016/j.jos.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimura N., Muraki S., Nakamura K., Tanaka S. Epidemiology of the locomotive syndrome: the research on osteoarthritis/osteoporosis against disability study 2005-2015. Mod Rheumatol. 2017;27:1–7. doi: 10.1080/14397595.2016.1226471. [DOI] [PubMed] [Google Scholar]
- 31.Wallace B.A., Cumming R.G. Systematic review of randomized trials of the effect of exercise on bone mass in pre- and postmenopausal women. Calcif Tissue Int. 2000;67:10–18. doi: 10.1007/s00223001089. [DOI] [PubMed] [Google Scholar]
- 32.Dalsky G.P., Stocke K.S., Ehsani A.A., Slatopolsky E., Lee W.C., Birge S.J., Jr. Weight-bearing exercise training and lumbar bone mineral content in postmenopausal women. Ann Intern Med. 1988;108:824–828. doi: 10.7326/0003-4819-108-6-824. [DOI] [PubMed] [Google Scholar]
- 33.Nelson M.E., Fisher E.C., Dilmanian F.A., Dallal G.E., Evans W.J. A 1-y walking program and increased dietary calcium in postmenopausal women: effects on bone. Am J Clin Nutr. 1991;53:1304–1311. doi: 10.1093/ajcn/53.5.1304. [DOI] [PubMed] [Google Scholar]
- 34.Hatori M., Hasegawa A., Adachi H., Shinozaki A., Hayashi R., Okano H. The effects of walking at the anaerobic threshold level on vertebral bone loss in postmenopausal women. Calcif Tissue Int. 1993;52:411–414. doi: 10.1007/BF00571327. [DOI] [PubMed] [Google Scholar]
- 35.Chien M.Y., Wu Y.T., Hsu A.T., Yang R.S., Lai J.S. Efficacy of a 24-week aerobic exercise program for osteopenic postmenopausal women. Calcif Tissue Int. 2000;67:443–448. doi: 10.1007/s002230001180. [DOI] [PubMed] [Google Scholar]
- 36.Zhang W., Nuki G., Moskowitz R.W., Abramson S., Altman R.D., Arden N.K. OARSI recommendations for the management of hip and knee osteoarthritis: part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18:476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 37.Anwer S., Alghadir A. Effect of isometric quadriceps exercise on muscle strength, pain, and function in patients with knee osteoarthritis: a randomized controlled study. J Phys Ther Sci. 2014;26:745–748. doi: 10.1589/jpts.26.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chou R., Huffman L.H., American Pain Society; American College of Physicians Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492–504. doi: 10.7326/0003-4819-147-7-200710020-00007. [DOI] [PubMed] [Google Scholar]
- 39.Sinaki M., Itoi E., Wahner H.W., Wollan P., Gelzcer R., Mullan B.P. Stronger back muscles reduce the incidence of vertebral fractures: a prospective 10 year follow-up of postmenopausal women. Bone. 2002;30:836–841. doi: 10.1016/s8756-3282(02)00739-1. [DOI] [PubMed] [Google Scholar]
- 40.Sakamoto K., Nakamura T., Hagino H., Endo N., Mori S., Muto Y. Effects of unipedal standing balance exercise on the prevention of falls and hip fracture among clinically defined high-risk elderly individuals: a randomized controlled trial. J Orthop Sci. 2006;11:467–472. doi: 10.1007/s00776-006-1057-2. [DOI] [PubMed] [Google Scholar]
- 41.Gillespie L.D., Robertson M.C., Gillespie W.J., Sherrington C., Gates S., Clemson L.M. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H.K., Suzuki T., Saito K., Yoshida H., Kobayashi H., Kato H. Effects of exercise and amino acid supplementation on body composition and physical function in community-dwelling elderly Japanese sarcopenic women: a randomized controlled trial. J Am Geriatr Soc. 2012;60:16–23. doi: 10.1111/j.1532-5415.2011.03776.x. [DOI] [PubMed] [Google Scholar]
- 43.Yokoyama Y., Nishi M., Murayama H., Amano H., Taniguchi Y., Nofuji Y. Association of dietary variety with body composition and physical function in community-dwelling elderly Japanese. J Nutr Health Aging. 2016;20:691–696. doi: 10.1007/s12603-015-0632-7. [DOI] [PubMed] [Google Scholar]
- 44.Yokoyama Y., Nishi M., Murayama H., Amano H., Taniguchi Y., Nofuji Y. Dietary variety and decline in lean mass and physical performance in community-dwelling older Japanese: a 4-year follow-up study. J Nutr Health Aging. 2017;21:11–16. doi: 10.1007/s12603-016-0726-x. [DOI] [PubMed] [Google Scholar]
- 45.Aoki K., Sakuma M., Ogisho N., Nakamura K., Chosa E., Endo N. The effects of self-directed home exercise with serial telephone contacts on physical functions and quality of life in elderly people at high risk of locomotor dysfunction. Acta Med Okayama. 2015;69:245–253. doi: 10.18926/AMO/53561. [DOI] [PubMed] [Google Scholar]
- 46.Schwanbeck S., Chilibeck P.D., Binsted G. A comparison of free weight squat to Smith machine squat using electromyography. J Strength Condit Res. 2009;23:2588–2591. doi: 10.1519/JSC.0b013e3181b1b181. [DOI] [PubMed] [Google Scholar]
- 47.Marchetti P.H., Jarbas da Silva J., Jon Schoenfeld B., Nardi P.S., Pecoraro S.L., D'Andréa Greve J.M. Muscle activation differs between three different knee joint-angle positions during a maximal isometric back squat exercise. J Sports Med (Hindawi Publ Corp) 2016;2016 doi: 10.1155/2016/3846123. 3846123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujiwara K., Toyama H., Asai H., Maeda K., Yaguchi C. Regular heel-raise training focused on the soleus for the elderly: evaluation of muscle thickness by ultrasound. J Physiol Anthropol. 2010;29:23–28. doi: 10.2114/jpa2.29.23. [DOI] [PubMed] [Google Scholar]
- 49.Suzuki T., Bean J.F., Fielding R.A. Muscle power of the ankle flexors predicts functional performance in community-dwelling older women. J Am Geriatr Soc. 2001;49:1161–1167. doi: 10.1046/j.1532-5415.2001.49232.x. [DOI] [PubMed] [Google Scholar]
- 50.Kurose S., Tsuyuguchi R., Tagashira S., Hamaguchi K., Shinno H., Seto T. Relationship between fall risk index and toe grip strength in the elderly. Jpn J Clin Sports Med. 2018;26:27–32. [Google Scholar]
- 51.Flanagan S.P., Wang M.Y., Greendale G.A., Azen S.P., Salem G.J. Biomechanical attributes of lunging activities for older adults. J Strength Condit Res. 2004;18:599–605. doi: 10.1519/1533-4287(2004)18<599:BAOLAF>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ohta M., Sasaki K., Sugita T., Takahashi A. Effects of locomotion training on locomotive syndrome in cases that could last for 6 months. J Jpn Soc Clin Sports Med. 2013;21:237–241. [Google Scholar]
- 53.Ishibashi H., Fujita H., Hosoi T., Arai T., Tokimura F., Anamizu Y. Verification of predictability of Locomotion Check on motor functions and effects of Locomotion Training on physical functions in the community-dwelling elderly. J Muscoskel Med. 2013;24:77–81. [Google Scholar]
- 54.Amao R., Otake Y., Ogata N., Haga N. Changes in physical ability and fall awareness by locomotion training for subjects with Locomotive syndrome. J Muscoskel Med. 2014;25:68–75. [Google Scholar]
- 55.Yasumura S., Hashimoto M. Intervention with the locomo-call strategy. Orthop Surg. 2013;64:1412–1415. [Google Scholar]
- 56.Niccoli S., Kolobov A., Bon T., Rafilovich S., Munro H., Tanner K. Whey protein supplementation improves rehabilitation outcomes in hospitalized geriatric patients: a double blinded, randomized controlled trial. J Nutr Gerontol Geriatr. 2017;36:149–165. doi: 10.1080/21551197.2017.1391732. [DOI] [PubMed] [Google Scholar]
- 57.Nabuco H.C.G., Tomeleri C.M., Sugihara Junior P., Fernandes R.R., Cavalcante E.F., Antunes M. Effects of whey protein supplementation pre- or post-resistance training on muscle mass, muscular strength, and functional capacity in pre-conditioned older women: a randomized clinical trial. Nutrients. 2018 May 3;10(5):E563. doi: 10.3390/nu10050563. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H., Suzuki T., Saito K., Kojima N., Hosoi E., Yoshida H. Long-term effects of exercise and amino acid supplementation on muscle mass, physical function and falls in community-dwelling elderly Japanese sarcopenic women: a 4-year follow-up study. Geriatr Gerontol Int. 2016;16:175–181. doi: 10.1111/ggi.12448. [DOI] [PubMed] [Google Scholar]
- 59.Hosoi T., Arai K., Orimo H. Questionnaire survey regarding osteoporosis screening and health education by local governments. Osteoporos Jpn. 2013;23:59–64. [Google Scholar]
- 60.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 61.Satake S., Shimada H., Yamada M., Kim H., Yoshida H., Gondo Y. Prevalence of frailty among community-dwellers and outpatients in Japan as defined by the Japanese version of the Cardiovascular Health Study criteria. Geriatr Gerontol Int. 2017;17:2629–2634. doi: 10.1111/ggi.13129. [DOI] [PubMed] [Google Scholar]