Abstract
Screening with FIT or colonoscopy can reduce CRC mortality. In our pragmatic, randomized trial of screening outreach over three years, patients annually received mailed FITs or colonoscopy invitations. We examined screening initiation after each mailing, and crossover from the invited to other modality. Eligible patients (50-64 years, ≥1 primary-care visit before randomization, and no history of CRC) received mailed FIT kits (n=2,400) or colonoscopy invitations (n=2,400) from March 2013 through July 2016. Among those invited for colonoscopy, we used multinomial logistic regression to identify factors associated with screening initiation with colonoscopy vs. FIT vs. no screening after the first mailing. Most patients were female (61.8%) and Hispanic (48.9%) or non-Hispanic black (24.0%). Among those invited for FIT, 56.6% (n=l,359) initiated with FIT, whereas 3.3% (n=78) crossed over to colonoscopy; 151 (15.7%) and 61 (7.7%) initiated with FIT after second and third mailings. Among those invited for colonoscopy, 25.5% (n=613) initiated with colonoscopy whereas 18.8% (n=452) crossed over to FIT; 112 (8.4%) and 48 (4.2%) initiated with colonoscopy after second and third mailings. Three or more primary-care visits prior to randomization was associated with initiating with colonoscopy (OR 1.49, 95% CI 1.17 – 1.91) and crossing over to FIT (OR 1.63, 95% CI 1.19 – 2.23). Although nearly half of patients initiated screening after the first mailing, few non-responders in either outreach group initiated after a second or third mailing. More patients invited to colonoscopy crossed over to FIT than those assigned to FIT crossed over to colonoscopy.
Keywords: colorectal neoplasms, mass screening, pragmatic clinical trial, health promotion, safety-net providers
Introduction
Screening for colorectal cancer (CRC) with fecal immunochemical tests (FIT) or colonoscopy can reduce mortality through prevention and early detection.1, 2 We conducted a large, pragmatic trial comparing population screening outreach strategies over a three-year period among primary-care patients in a safety-net healthcare system.3, 4 To understand how the outreach strategies worked among various patient subgroups, and what outcomes to expect with wider-spread implementation, we addressed three research questions:
How many patients initiated screening or crossed over after each of the three outreach mailings?
Among non-responders to the first mailing, how many patients initiated following the second and third mailings (i.e., intervention doses)?
Among responders to the first mailing, what factors were associated with initiating screening with the invited modality vs. crossing over to the other modality?
Methods
We conducted post-hoc analyses of data collected from a pragmatic trial of CRC screening outreach (Clinicaltrials.gov identifier: NCT01710215). Details of the study design, recruitment, and findings are reported elsewhere.3, 4
Patients not up-to-date with CRC screening5 were randomly assigned between March 2013 and January 2014 to receive usual care or one of two outreach strategies: mailed invitation to complete an enclosed FIT or mailed invitation to schedule and complete colonoscopy. Study materials were in Spanish and English, and all study staff were bilingual. Both outreach groups received up to two reminder calls 2-3 weeks after the mailings. FIT invitations were sent every 12 months; patients who did not complete colonoscopy received repeat invitations each year. Usual-care participants received visit-based screening at the discretion of primary-care providers. Follow-up continued until July 2016. Compared to usual care, screening completion over the three-year period was higher for both outreach groups (FIT outreach, 28.0%; colonoscopy outreach, 38.4%) and highest for colonoscopy outreach (38.4% vs. 10.7% in usual care).3
We extend findings of the trial by examining the proportion of patients initiating screening after multiple outreach mailings. For these analyses, we included participants assigned to FIT (n=2,400) or colonoscopy (n=2,400) outreach.
Statistical analysis
Separately for each group, we estimated proportions who initiated screening after the first, second, and third outreach mailing. We also examined crossover from the invited to the other screening modality. For example, among patients invited for colonoscopy, we report proportions initiating screening with colonoscopy and who crossed over to initiate screening with FIT.
We then examined differences in characteristics of patients initiating screening with the invited modality vs. crossing over to initiate with the other modality. Among patients invited to colonoscopy (n=2,400), we identified correlates of screening initiation with colonoscopy vs. crossing over to FIT, with “not screened” as the referent category. We built a mixed-effects multinomial logistic regression model6 to account for the nested structure of patients within clinics (n=8 clinics). We limited this analysis to patients assigned to colonoscopy outreach because few patients invited to FIT crossed over to colonoscopy.
All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC). The study was approved by the Institutional Review Board at UT Southwestern Medical Center (#102011-069).
Results
Among patients assigned to FIT outreach, 56.6% (n=l,359) initiated with FIT after the first mailing, and 3.3% (n=78) crossed over to colonoscopy (Figure 1). An additional 151 (15.7%) and 61 (7.7%) of non-responders initiated screening with FIT after the second and third mailings, respectively. About one-third (n=724, 30.2%) of patients invited for FIT never initiated screening. Compared to other racial/ethnic groups, more Hispanics (64.2%) and patients with Spanish as their primary language (66.9%) initiated FIT (Table 1). More patients with comorbidity scores ≥2 (4.5% vs. 1.9% with a score=1), ≥3 primary care visits prior to randomization (4.2% vs. 2.1% with 1 visit), and who received care in academic clinics (4.9%) versus neighborhood clinics (3.0%), crossed over to colonoscopy.
Figure 1.
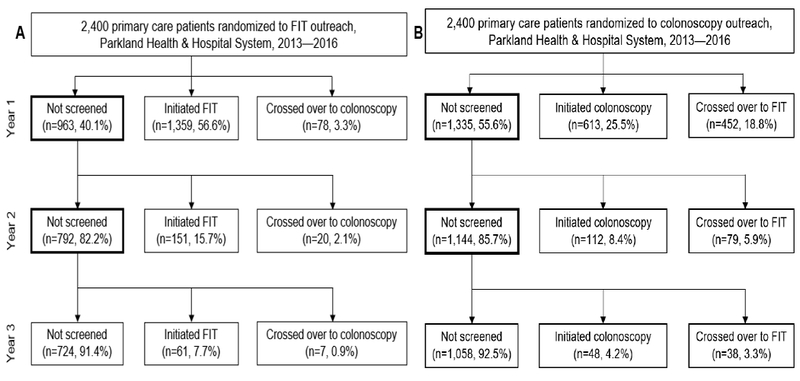
Study flow diagram showing screening initiation and cross over after three outreach mailings among participants invited for FIT (A; n=2,400) and colonoscopy (B; n=2,400), Parkland Health & Hospital System, 2013 – 2016
Table 1.
Characteristics of patients by outreach group and screening status at Year 1: not screened, initiated invited screening modality, and crossed over to different screening modality (n=4,800)
| FIT outreach (n==2,400) | Colonoscopy outreach (n=2,400) | |||||
|---|---|---|---|---|---|---|
| Not screened (n=963) | Initiated FIT (n=1,359) | Crossed over to colonoscopy (n=78) | Not screened (n=1,335) | Initiated Colonoscopy (n=613) | Crossed over to FIT (n=452) | |
| Age | ||||||
| 50-54 | 391 (40.4) | 542 (56.0) | 35 (3.6) | 575 (55.9) | 272 (26.5) | 181 (17.6) |
| 55-59 | 325 (40.2) | 456 (56.4) | 27 (3.3) | 427 (55.7) | 196 (25.6) | 143 (18.7) |
| 60-64 | 247 (39.6) | 361 (57.9) | 16 (2.6) | 333 (55.0) | 145 (23.9) | 128 (21.1) |
| Sex | ||||||
| Male | 565 (38.3) | 868 (58.9) | 41 (2.8) | 805 (53.9) | 385 (25.8) | 304 (20.4) |
| Female | 398 (43.0) | 491 (53.0) | 37 (4.0) | 530 (58.5) | 228 (25.2) | 148 (16.3) |
| Race/ethnicity | ||||||
| Non-Hispanic white | 268 (52.1) | 230 (44.8) | 16 (3.1) | 361 (66.9) | 100 (18.5) | 79 (14.6) |
| Non-Hispanic black | 270 (45.5) | 308 (51.9) | 16 (2.7) | 339 (60.8) | 120 (21.5) | 99 (17.7) |
| Hispanic | 375 (32.3) | 746 (64.2) | 41 (3.5) | 571 (48.2) | 361 (30.5) | 252 (21.3) |
| Other | 50 (38.5) | 75 (57.7) | 5 (3.9) | 64 (54.2) | 32 (27.1) | 22 (18.6) |
| Comorbidity score | ||||||
| 0 | 429 (37.1) | 681 (59.0) | 45 (3.9) | 628 (54.6) | 318 (27.6) | 205 (17.8) |
| 1 | 362 (40.8) | 508 (57.3) | 17 (1.9) | 492 (54.6) | 212 (23.5) | 197 (21.9) |
| 2+ | 172 (48.0) | 170 (47.5) | 16 (4.5) | 215 (61.8) | 83 (23.9) | 50 (14.4) |
| Primary care visits prior to randomization | ||||||
| 1 | 392 (41.5) | 533 (56.4) | 20 (2.1) | 586 (58.8) | 234 (23.5) | 176 (17.7) |
| 2 | 268 (42.0) | 346 (54.2) | 24 (3.8) | 345 (54.7) | 170 (26.9) | 116 (18.4) |
| 3+ | 303 (37.1) | 480 (58.8) | 34 (4.2) | 404 (52.3) | 209 (27.0) | 160 (20.7) |
| Clinic type | ||||||
| Neighborhood | 835 (39.5) | 1215 (57.5) | 64 (3.0) | 1161 (54.5) | 541 (25.4) | 428 (20.1) |
| Academic | 128 (44.8) | 144 (50.4) | 14 (4.9) | 174 (64.4) | 72 (26.7) | 24 (8.9) |
Among those assigned to colonoscopy outreach, 25.5% (n=613) initiated screening with colonoscopy after the first mailing, and 18.8% (n=452) crossed over to FIT. An additional 112 (8.4%) and 48 (4.2%) of non-responders initiated screening with colonoscopy after the second and third mailing, respectively. Almost half of patients (n=l,058, 44.1%) never initiated screening. A higher proportion of Hispanics (51.8% vs. 33.1% of non-Hispanic whites) and patients receiving care in neighborhood clinics (45.5% vs. 35.5% in academic clinics) initiated any screening (Table 1). More 60-64 year olds (21.1%), Hispanics (21.3%), and patients with Spanish as their primary language (22.6%) crossed over to FIT.
Multinomial logistic regression analysis (Supplementary Table 1) showed clinic type (academic clinic, OR 0.35, 95% CI 0.21 – 0.59), ≥3 primary-care visits prior to randomization (OR 1.63, 95% CI 1.19 – 2.23), and Hispanic race/ethnicity (OR 2.06, 95% CI 1.47 – 2.89) were associated with crossing over to FIT (vs. not screened).
Discussion
Our findings suggest outreach strategies either worked as intended, by engaging patients outside of primary care, or by prompting patients to discuss screening with primary-care providers and perhaps cross over to a different test. More patients assigned to colonoscopy outreach crossed over to FIT than those assigned to FIT crossed over to colonoscopy. Those invited for colonoscopy but crossed over to FIT had to have done so through their primary-care provider because FIT kits were not sent with colonoscopy invitations. About 20% of patients in neighborhood clinics crossed over to FIT, which may reflect physician preferences and recommendations.7
Nearly half of patents initiated screening after the first outreach mailing, but few initiated after a second or third mailing. These results challenge conventional wisdom that some hesitant patients need two or three intervention doses to initiate screening8 and instead raise questions concerning the benefit of and incremental cost of additional doses.8 Delivery of a second intervention dose to more than 2,000 non-responders resulted in only about 360 additional persons screened (170 FIT, 190 colonoscopy). Yield was even lower for a third mailing — only 150 of 1,900 remaining non-responders initiated screening. Although some patients may have moved away from the healthcare system or changed addresses, the number of non-responders exceeds the number lost to follow-up.3 These findings add to results from other cancer screening interventions that have shown small or no benefit of delivering booster materials to participants who do not become adherent after the first dose.9 Given the low yield and incremental cost of additional doses, identifying alternative strategies for re-contacting and inviting non-responders, and understanding reasons for non-response, may improve screening initiation more than delivering the same outreach invitation.
With the exception of clinic type, findings from our multinomial model provides little insight about which patients cross over to a different screening modality. Patient preferences - not captured in our study - may more strongly predict cross over than sociodemographic characteristics. A number of studies have described patient preferences for CRC screening,10–14 generally demonstrating preference for the test patients feel most confident in completing. Others have shown that, compared to patients invited for colonoscopy, patients who receive recommendations for stool testing or are offered a choice between stool tests or colonoscopy are more likely to initiate screening with a stool test.15 Incorporating preferences in mailed outreach materials may increase likelihood of screening initiation with a test of patients’ choice.
In summary, findings from a large, pragmatic trial of CRC screening outreach show many patients crossed over to a screening test other than the one offered through outreach. Few non-responders initiated screening after a second or third dose of the same intervention. Although these findings are generalizable only to outreach programs similar to those tested in our trial, they provide insight into how best to optimize programs and manage population health. In the future, outreach programs should understand the optimal intensity and incremental value of mailed invitations over time, as well as reasons for non-response. Considering offering non-responders alternative interventions may optimize healthcare system efforts to implement population-based cancer screening programs.
Supplementary Material
Highlights.
About 50% initiated FIT and 25% initiated colonoscopy after the first mailing
Very few non-responders initiated screening after a second or third mailing
About 20% of patients invited for colonoscopy crossed over to FIT
Screening initiation was associated with race/ethnicity and primary care visits
Acknowledgments
A version of this manuscript was presented at Digestive Disease Week 2018, Washington, D.C., June 2018.
This work was supported by the National Cancer Institute (U54 CA163308, P30 CA142543) and National Center for Advancing Translational Sciences (KL2 TR001103 to Dr. Murphy) at the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
The sponsors had no role in: design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Research support: National Cancer Institute (P30 CA142543, U54 CA163308) and National Center for Advancing Translational Sciences (KL2 TR001103) at the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors report no conflicts of interest or financial disclosures.
Guarantor of the article:
Dr. Murphy had full access to all the data in the study and takes responsibility for the data and the accuracy of the data analysis.
Conflicts of interest and financial disclosures: The authors declare no conflicts of interest or financial disclosures.
References
- 1.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, et al. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2008;149:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudsen AB, Zauber AG, Rutter CM, et al. Estimation of Benefits, Burden, and Harms of Colorectal Cancer Screening Strategies: Modeling Study for the US Preventive Services Task Force. Jama 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singal AG, Gupta S, Skinner CS, et al. Effect of Colonoscopy Outreach vs Fecal Immunochemical Test Outreach on Colorectal Cancer Screening Completion: A Randomized Clinical Trial. Jama 2017;318:806–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singal AG, Gupta S, Tiro JA, et al. Outreach invitations for FIT and colonoscopy improve colorectal cancer screening rates: A randomized controlled trial in a safety-net health system. Cancer 2016;122:456–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Jama 2016;315:2564–2575. [DOI] [PubMed] [Google Scholar]
- 6.Hedeker D A mixed-effects multinomial logistic regression model. Stat Med 2003;22:1433–46. [DOI] [PubMed] [Google Scholar]
- 7.Klabunde CN, Lanier D, Nadel MR, et al. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006-2007. Am J Prev Med 2009;37:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skinner CS, Kobrin SC, Monahan PO, et al. Tailored interventions for screening mammography among a sample of initially non-adherent women: when is a booster dose important? Patient Educ Couns 2007;65:87–94. [DOI] [PubMed] [Google Scholar]
- 9.Rakowski W, Lipkus IM, Clark MA, et al. Reminder letter, tailored stepped-care, and self-choice comparison for repeat mammography. Am J Prev Med 2003;25:308–14. [DOI] [PubMed] [Google Scholar]
- 10.Hawley ST, McQueen A, Bartholomew LK, et al. Preferences for colorectal cancer screening tests and screening test use in a large multispecialty primary care practice. Cancer 2012;118:2726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hawley ST, Vernon SW, Levin B, et al. Prevalence of colorectal cancer screening in a large medical organization. Cancer Epidemiol Biomarkers Prev 2004;13:314–9. [DOI] [PubMed] [Google Scholar]
- 12.Hawley ST, Volk RJ, Krishnamurthy P, et al. Preferences for colorectal cancer screening among racially/ethnically diverse primary care patients. Med Care 2008;46:S10–6. [DOI] [PubMed] [Google Scholar]
- 13.Powell AA, Burgess DJ, Vernon SW, et al. Colorectal cancer screening mode preferences among US veterans. Prev Med 2009;49:442–8. [DOI] [PubMed] [Google Scholar]
- 14.Jimbo M, Sen A, Plegue MA, et al. Correlates of Patient Intent and Preference on Colorectal Cancer Screening. Am J Prev Med 2017;52:443–450. [DOI] [PubMed] [Google Scholar]
- 15.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: a randomized clinical trial of competing strategies. Arch Intern Med 2012;172:575–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


