Abstract
Background:
Single-nucleotide polymorphisms (SNPs) associated with melanoma have been identified though genome-wide association studies. However, the combined impact of these SNPs on melanoma development remains unclear, particularly in postmenopausal women who carry a lower melanoma risk.
Objective:
We examine the contribution of a combined polygenic risk score on melanoma development in postmenopausal women.
Methods:
Genetic risk scores were calculated using 21 genome-wide association study—significant SNPs. Their combined effect on melanoma development was evaluated in 19,102 postmenopausal white women in the clinical trial and observational study arms of the Women’s Health Initiative dataset.
Results:
Compared to the tertile of weighted genetic risk score with the lowest genetic risk, the women in the tertile with the highest genetic risk were 1.9 times more likely to develop melanoma (95% confidence interval 1.50-2.42). The incremental change in c-index from adding genetic risk scores to age were 0.075 (95% confidence interval 0.041-0.109) for incident melanoma.
Limitations:
Limitations include a lack of information on nevi count, Fitzpatrick skin type, family history of melanoma, and potential reporting and selection bias in the Women’s Health Initiative cohort.
Conclusion:
Higher genetic risk is associated with increased melanoma prevalence and incidence in postmenopausal women, but current genetic information may have a limited role in risk prediction when phenotypic information is available.
Keywords: genetic risk score, melanoma, postmenopausal, single-nucleotide polymorphism, women, Women’s Health Initiative
CAPSULE SUMMARY
• Pooling the effects of single nucleotide polymorphisms as genetic risk scores can increase their clinical utility.
• The highest genetic risk score is associated with increased melanoma prevalence and incidence in postmenopausal women with modest disease predictive ability.
• Polygenic risk scores have limited utility for melanoma prediction when phenotypic data are available.
Cutaneous melanoma is a leading cause of skin cancer mortality, with as high as 55% of variation in melanoma risk attributable to genetic influences.1 Single nucleotide polymorphisms (SNPs) contribute a low risk of melanoma individually; however, pooling their effects as a combined genetic risk score (GRS) can increase utility. A recent study involving 8950 participants from MD Anderson and the Nurses’ Health Study found that individuals in the tertile of highest melanoma genetic risk, calculated using combined information from 11 SNPs, were 2.13 times more likely to have melanoma when compared to those in the tertile of lowest risk.2 A recent case-control study by Kypreou et al3 affirmed the similar effect size of another polygenic risk score (PRS), calculated from 15 SNPs, in a Southern Greek population. Both studies demonstrated the incremental benefit of using a combined PRS in predicting melanoma versus information from a single locus. For instance, in the study using MD Anderson and Nurses’ Health Study databases, the most significant SNP, rs12913832, had an odds ratio (OR) of 1.29 (95% confidence interval [CI] 1.12-1.48) compared to an OR of 2.13 in a PRS pooling 11 SNPs. While higher genetic risk is significantly associated with increased melanoma events, the predictive ability of these SNPs in melanoma development and their clinical relevance in other cohorts are not well characterized.
Female sex has been associated with lower melanoma risk across all age groups and health care delivery models.4 Hormonal differences have been postulated to be a key contributor to this sex difference; however, lower melanoma risk and improved prognosis continue to persist in postmenopausal women.5 Postmenopausal women are an interesting population to study melanoma risk because they have a lower risk of melanoma but do not have confounding hormonal influences that are as significant. A previous study by the National Cancer Institute found that phenotypic modeling of pigmentation and sun exposure had lower predictive power for melanoma in women ≥50 years of age compared to men.6 This may be because of a combination of environmental factors and sex-associated genetic differences.7 The aim of this study is to assess the combined impact of melanoma risk SNPs and their additive effects when combined with phenotypic factors in a melanoma prediction model using a longitudinal cohort of 161,808 postmenopausal women in the clinical trial (CT) and observational study (OS) arms of the Women’s Health Initiative (WHI) database.8 This study expands upon the existing literature by assessing the impact of an updated list of 21 SNPs9 in melanoma risk, focusing on postmenopausal women who have better melanoma prognosis compared to general mixed population in previous studies, and including prospective risk as reflected by incident melanoma, compared to previous retrospective case-control study designs.
METHODS
Cohort identification in the WHI dataset
Of 161,808 postmenopausal women in the CT and OS arms of the WHI dataset, a total of 19,102 white women were genotyped (Supplemental Methods, available online at http://www.jaad.org), including 422 lifetime melanoma cases and 289 incident melanoma cases encompassing a median of 16.8 follow-up years (Supplemental Fig 1, available online at http://www.jaad.org). Lifetime melanoma cases were self-reported in surveys and included those with a history of melanoma at the time of the enrollment. Incident melanoma cases were identified from medical records and confirmed by histology after the study enrollment. They also excluded those with a history of melanoma at the time of the enrollment. When considering incident melanoma, women who did not report melanoma were censored at the date of their last follow-up, date of death, or September 30, 2015 for women who were still being followed. The study was approved by the Stanford Institutional Review Board.
GRS calculation
We generated a GRS for each woman using 21 SNPs reported in the published literature,9 which represent the most updated list of genome-wide significant SNPs as of January 22, 2018 (published on August 5, 2015) since the previous GRS studies. We divided women into equivalently sized tertiles of highest genetic risk, normal genetic risk, and lowest genetic risk, following the approach of previous studies in melanoma, diabetes, and cardiovascular disease.2,10,11 Beta coefficients of 21 SNPs reported in published literature were used as weights and the following formula was applied to calculate the GRS:
Where EA is an indicator for the presence of effect alleles at allele k at SNP s (s = 0,1) and Ws represents the beta coefficient estimates of effect allele of SNPs from the published literature. We did not compare the impact of assigning different weights to each allele in GRS calculation.
Statistical analyses of the association between GRS and melanoma
To estimate the association between GRS and lifetime and incident melanoma, logistic regression and Cox proportional hazards regression models were performed, respectively. Both models included tertiles of GRS as the independent variable of interest and adjusted for age. To assess proportional hazard assumptions, a Kaplan—Meier plot was visually examined. Some of the covariates had missing values, and therefore 5 multiple imputations by chained equations using the Multivariate Imputation via Chained Equations (MICE) package (R software, version 2.46.0).
C-index and area under the curve calculations
The c-index and area under the curve (AUC) were estimated to assess the performance of the predictive capability of GRS for incident melanoma and lifetime melanoma. AUC is a plot of the true positive rate on a Y axis and false positive rate on an X axis, and c-index is a numerical measure of AUC and reflects the goodness of fit in survival models of the plot. A c-index of 0.5 represents the goodness of fit of random, with an equal chance of true negative and true positive events. A c-index <0.5 represents a fit worse than random, with a higher likelihood of true negative and a poor predictive model. Conversely, a c-index >0.5 represents a fit better than random, with a higher likelihood of true positive and positive predictive capacity. Additional details are described in the Supplement.
Sensitivity analyses
In sensitivity analyses, we included additional covariates in the model, such as sunscreen use, skin type, and latitude. Because those additional covariates were only collected in the OS cohort and we observed few cases of melanoma, we performed the sensitivity analyses on the OS cohort and we adjusted the model for 1 additional variable at a time. The study was underpowered to adjust for all variables together because of the limited number of melanoma cases. All statistical analyses were performed with R software (version 3.1.3).12 All tests were 2-sided and conducted at the 0.05 significance level, and all model estimates are shown with 95% confidence intervals.
RESULTS
The median age of the CT-OS cohort was 67 years old, and approximately 48% of the subjects resided in northern latitudes (Supplemental Table I, available online at http://www.jaad.org). The CT-OS cohort included 422 cases of lifetime melanoma and 289 cases of incident melanoma (Supplemental Fig 1). A subset of 3146 women in the OS cohort also had additional information on skin type and sunscreen use (Supplemental Table II, available online at http://www.jaad.org). In the CT-OS cohort, region and latitude of residence, medication usage, age, smoking status, and body mass index were comparable across all GRS tertiles (Supplemental Tables I and II). Twenty-one SNPs that demonstrated genome-wide significance in previous studies (Supplemental Table III, available online at http://www.jaad.org) were included in the GRS calculation. GRS was calculated using the risk alleles and beta coefficients of SNPs published in the literature as weights, which was calculated using the risk alleles and betas that were defined according to previous reports3 (Supplement, available online at http://www.jaad.org).
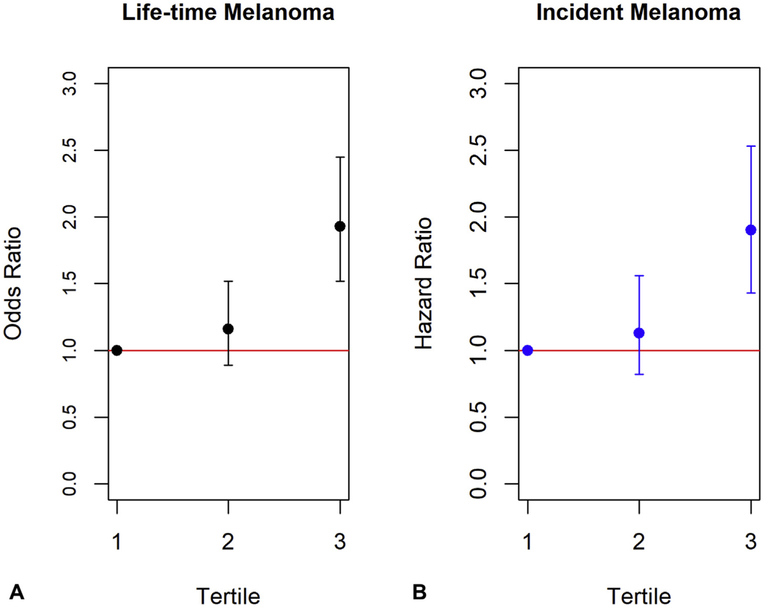
To compare the weighted GRS across the tertiles for lifetime risk of melanoma, a logistic regression model was created, adjusting for age and tertiles of weighted GRS. Women in the tertile with highest genetic risk were 1.91 times more likely to have lifetime melanoma than women in the tertile with lowest genetic risk (95% CI 1.50-2.42) (Fig 1, A). A similar increase in risk was observed for incident melanoma during >20 years of follow-up, with a hazard ratio of 1.89 for the highest tertile compared to the lowest tertile (95% CI 1.42-2.52; Fig 1, B and Supplemental Fig 2, available online at http://www.jaad.org). The effect size in this study was consistent with those reported previously in Fang et al’s study.2 These data support the conclusion that higher GRS scores are associated with increased melanoma prevalence and incidence in postmenopausal women.
Fig 1.
Risk of lifetime and incident melanoma by genetic risk score (GRS). A, Logistic regression model adjusted for age and tertiles of weighted GRS demonstrate increased risk of lifetime melanoma with an odds ratio of 1.91 (95% confidence interval 1.50-2.42, P < .01) in the highest GRS tertile compared to the lowest tertile. B, Cox proportional hazards regression model adjusting for the same covariates demonstrate an increased risk of incident melanoma with a similar hazard ratio of 1.89 (95% confidence interval 1.42-2.52, P < .01) in the highest tertile compared to the lowest.
Because the covariates considered in the primary regression models did not include information on skin type, sunscreen use, or latitude of residence, we conducted a second sensitivity analysis using only the OS cohort. Compared with the CT-OS cohort, the highest GRS tertile in the OS cohort were 2.89 times more likely to develop lifetime melanoma compared to the lowest tertile (95% CI 1.77-4.71). Adjusting for skin type, sunscreen use, or latitude, the odds ratio for lifetime melanoma continued to remain statistically significant at P < .01, with odds ratios of 2.64 (95% CI 1.61-4.34), 2.72 (95% CI 1.66-4.45), and 2.92 (95% CI 1.79-4.78), respectively, when comparing the melanoma risk in the highest genetic risk tertile to the lowest genetic risk tertile (Supplemental Table IV, available online at http://www.jaad.org). Similar results were observed for incident melanomas, with odds ratio of 2.65 (95% CI 1.17-6.01), 2.72 (95% CI 1.20-6.16), and 3.00 (95% CI 1.33-6.78), respectively (Supplemental Table V, available online at http://www.jaad.org). The sensitivity analyses using the OS cohort supported the association between the highest genetic risk tertile and increased melanoma risk even when adjusting for sun exposure data.
The c-index of phenotypic model with age only for incident melanoma was 0.532 (95% CI 0.498-0.566), which was comparable to that of phenotypic model with sex and age alone.2 By adding GRS to the model, the c-index was increased by modest amount of 0.068 (95% CI 0.034-0.102) for incident melanoma. The AUC of the phenotypic model for lifetime melanoma was 0.517 (95% CI 0.489-0.545), and the difference was 0.075 (95% CI 0.041-0.109; Table I). The predictive power of the phenotypic model in our study was lower relative to previous studies, likely because of the absence of nevi data in the model. Consistent with the previous studies, the interval increase from addition of phenotypic model to GRS model was modest.
Table I.
Predictive ability of phenotypic and genetic risk score models in the Women’s Health Initiative dataset
| Predictive models | C-index/AUC* (95% CI) |
|---|---|
| Lifetime melanoma | |
| Age only | 0.517 (0.489–0.545) |
| Incremental increase from addition of GRS | 0.075 (0.041–0.109) |
| Incident melanoma | |
| Age only | 0.532 (0.498–0.566) |
| Incremental increase from addition of GRS | 0.068 (0.034–0.102) |
AUC, Area under the curve; CI, confidence interval; GRS, genetic risk score.
C-index calculated for incident melanoma using survival model. AUC calculated for lifetime melanoma using logistic regression.
CONCLUSIONS
In this study, we demonstrated the combined impact of a PRS on the development of melanoma in a large longitudinal cohort of postmenopausal women. Consistent with studies conducted in other populations, the top GRS tertile in the WHI cohort of postmenopausal women were 1.91 times more likely to have lifetime melanoma when compared to the lowest tertile. The present study additionally confirmed this increased association in incident melanoma as well, with the top tertile 1.89 times more likely to have an event when compared to the lowest. The conclusions of this study bring attention to two interesting points. First, this confirms the relevance of currently known SNPs associated with melanoma, whose studies have been previously limited to mixed populations of male and females, in a population of postmenopausal women. This is important because postmenopausal women have been suggested to have some difference in melanoma biology compared to men. Second, the modest impact of genetic risk in this study is an interesting example of how pooled genetic risk can have minimal impact overall on the actual clinical development of melanoma despite associated risk. This may suggest that environmental factors may have more significant role in melanoma development compared to genetic factors than previously suspected.
The strength of the study is in the robust longitudinal phenotypic data available in the WHI database that allows assessment of lifetime and incident melanoma, including skin type and sunscreen use. A limitation of the study is the lack information on the additional phenotypic factors, including nevi count, Fitzpatrick skin type, and family history of melanoma. Other limitations include potential reporting bias in self-reported lifetime melanoma and selection bias in the subet of WHI cohort who had genetic information available. Our findings revealed a predictive effect of genetic factors for melanoma in an older female population, which was attenuated when phenotypic factors were incorporated. It is important to recognize the likelihood of some overlap in the phenotypic factors and genetic risk. For instance, SNPs in genes known to affect skin type and nevi characteristics, such as MC1R and TYR, likely lack additional predictive capcity when combined with phenotypic factors in a disease prediction model as their addition adds redundant information to the prediction model.
The clinical utility of PRS have been also studied, both alone and in combination with phenotypic factors in areas outside of cutaneous oncology, including cardiovascular disease, such as coronary artery disease and artrial fibrillation,13,14 age-associated Alzheimer disease,15 and diabetes.16 These studies have also found a predictive impact of GRS that is attenuated when phenotypic information is incorporated. Our study highlights the need to further identify rare high-impact SNPs in melanoma and suggests that currently PRSs have limited utility for melanoma risk when clinical phenotypic data are available.
Women’s Health Initiative investigators included the following at the program office (National Heart, Lung, and Blood Institute, Bethesda, MD): Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller, and at the Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg. Investigators and Academic Centers included JoAnn E. Manson (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA); Barbara V. Howard (MedStar Health Research Institute/Howard University, Washington, DC); Marcia L. Stefanick (Stanford Prevention Research Center, Stanford, CA); Rebecca Jackson (The Ohio State University, Columbus, OH); Cynthia A. Thomson (University of Arizona, Tucson/Phoenix, AZ); Jean Wactawski-Wende (University at Buffalo, Buffalo, NY); Marian Limacher (University of Florida, Gainesville/Jacksonville, FL); Jennifer Robinson (University of Iowa, Iowa City/ Davenport, IA); Lewis Kuller (University of Pittsburgh, Pittsburgh, PA); Sally Shumaker (Wake Forest University School of Medicine, Winston-Salem, NC); Robert Brunner (University of Nevada, Reno, NV); and Karen L. Margolis (University of Minnesota, Minneapolis, MN). The Women’s Health Initiative Memory Study was conducted by Mark Espeland (Wake Forest University School of Medicine, Winston-Salem, NC).
Supplementary Material
Acknowledgments
Supported by a Dermatology Foundation Grant (Dr Sarin) and the Stanford Medical Scholars Research Program (Ms Cho). The Women’s Health Initiative is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, and the US Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN 268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
Conflicts of interest: None disclosed.
REFERENCES
- 1.Shekar SN, Duffy DL, Youl P, et al. A population-based study of Australian twins with melanoma suggests a strong genetic contribution to liability. J Invest Dermatol. 2009;129: 2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fang S, Han J, Zhang M, et al. Joint effect of multiple common SNPs predicts melanoma susceptibility. PLoS One. 2013;8: e85642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kypreou KP, Stefanaki I, Antonopoulou K, et al. Prediction of melanoma risk in a southern European population based on a weighted genetic risk score. J Invest Dermatol. 2016;136:690–695. [DOI] [PubMed] [Google Scholar]
- 4.Voinea S, Bildaru A, Panaitescu E, Sandru A. Impact of gender and primary tumor location on outcome of patients with cutaneous melanoma. J Med Life. 2016;9:444–448. [PMC free article] [PubMed] [Google Scholar]
- 5.Enninga EAL, Moser JC, Weaver AL, et al. Survival of cutaneous melanoma based on sex, age, and stage in the United States, 1992–2011. Cancer Med. 2017;6:2203–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fears TR, Guerry DT, Pfeiffer RM, et al. Identifying individuals at high risk of melanoma: a practical predictor of absolute risk. J Clin Oncol. 2006;24:3590–3596. [DOI] [PubMed] [Google Scholar]
- 7.Hernando B, Ibarrola-Villava M, Fernandez LP, et al. Sex-specific genetic effects associated with pigmentation, sensitivity to sunlight, and melanoma in a population of Spanish origin. Biol Sex Differ. 2016;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 9.Law MH, Bishop DT, Lee JE, et al. Genome-wide meta-analysis identifies five new susceptibility loci for cutaneous malignant melanoma. Nat Genet. 2015;47:987–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim NK, Lee JY, Lee JY, Park HY, Cho MC. The role of genetic risk score in predicting the risk of hypertension in the Korean population: Korean Genome and Epidemiology Study. PLoS One. 2015;10:e0131603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villegas R, Delahanty R, Gao YT, et al. Joint effect of genetic and lifestyle risk factors on type 2 diabetes risk among Chinese men and women. PLoS One. 2012;7:e49464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria; 2014. [Google Scholar]
- 13.Svensson T, Kitlinski M, Engstrom G, Melander O. A genetic risk score for CAD, psychological stress, and their interaction as predictors of CAD, fatal MI, non-fatal MI and cardiovascular death. PLoS One. 2017;12:e0176029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lubitz SA, Yin X, Lin HJ, et al. Genetic risk prediction of atrial fibrillation. Circulation. 2017;135:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Desikan RS, Fan CC, Wang Y, et al. Genetic assessment of age-associated Alzheimer disease risk: development and validation of a polygenic hazard score. Plos Med. 2017;14: e1002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emdin CA, Khera AV, Natarajan P, et al. Genetic association of waist-to-hip ratio with cardiometabolic traits, type 2 diabetes, and coronary heart disease. JAMA. 2017;317:626–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.