Figure 1.
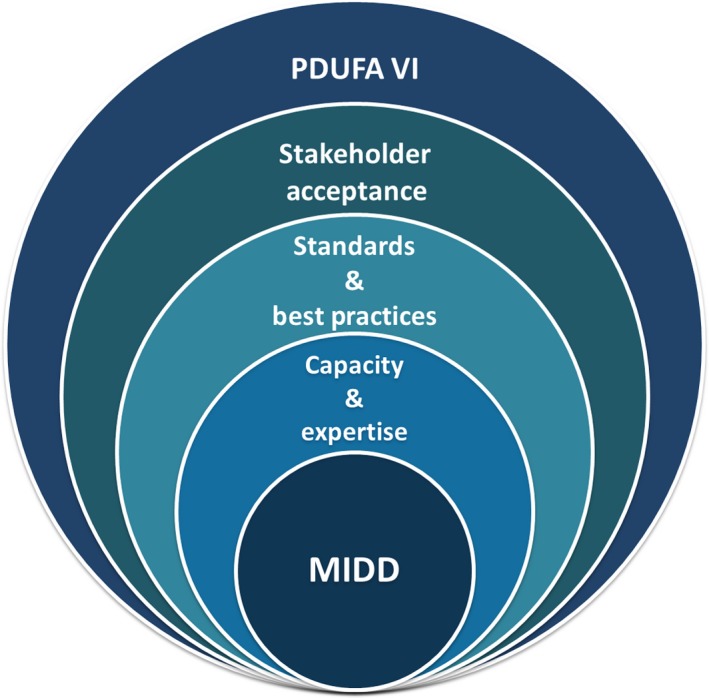
An integrative approach for advancing model‐informed drug development (MIDD) under Prescription Drug User Fee Act (PDUFA) VI. MIDD can be seen as foundational to efficient and effective drug development and regulatory evaluation of small molecule drugs and biological products. To advance more widespread and predictable application, MIDD requires an adequate staff capacity and expertise, community‐accepted standards and best practices, and multistakeholder acceptance eyond technical experts. The commitments laid out under PDUFA VI provide an opportunity to achieve these goals in a holistic and integrated manner.
