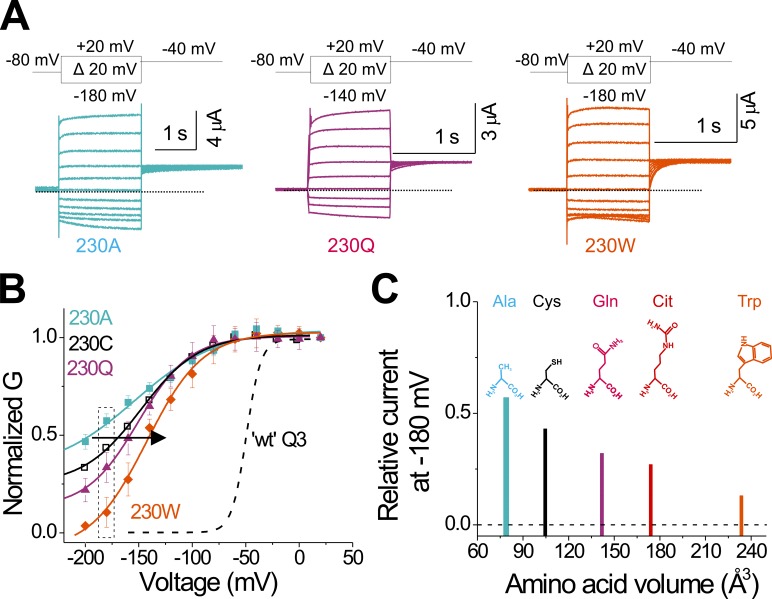
Figure 5.
The reduction of the side chain size at position 230 contributes to the leftward shift in the G(V). (A) Representative current traces from KCNQ3-A315T-R230A (light blue), KCNQ3-A315T-R230Q (magenta), and KCNQ3-A315T-R230W (orange) for the indicated voltage protocol. (B) Extrapolated tail conductances from R230x mutations from A were normalized (G(V), see Materials and methods) and plotted versus test voltages (means ± SEM; n = 8–11). Lines represent the fitted theoretical voltage dependencies (see Materials and methods, Eqs. 1 and 2). Dashed line represents the G(V) curve of KCNQ3-A315T (WT Q3) for comparison. Dotted lines represent zero current. (C) Relative current at −180 mV from R230x mutations taken from dashed rectangle in B, plotted against amino acid volume in aqueous solution (Zamyatnin, 1972). Dashed line represents the current of WT KCNQ3-A315T-R230R at −180 mV. Structure of the natural amino acids (A, C, Q, and W) and the unnatural amino acid citrulline (Cit), the uncharged close structural analogue of arginine, are shown for comparison. Citrulline volume is estimated from that of arginine. The same color code for the different KCNQ3 bearing R230x amino acid substitutions is shown throughout the figure.