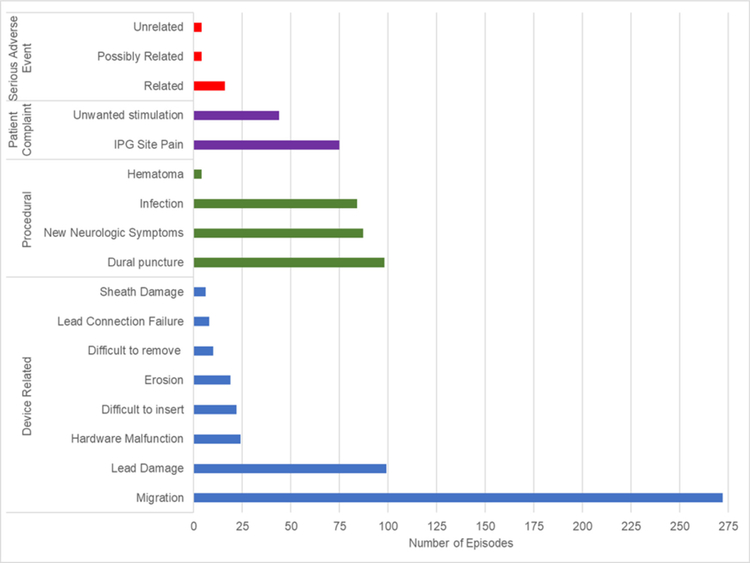
Figure 3.
Subcategorization of device-related, procedural, patient complaint, and serious adverse event complication categories. *If multiple complications occurred for the same episode, each was recorded each as a separate complication. If a particular complication was considered serious and managed via a separately recorded category, it was reported in both categories. IPG, implantable pulse generator.