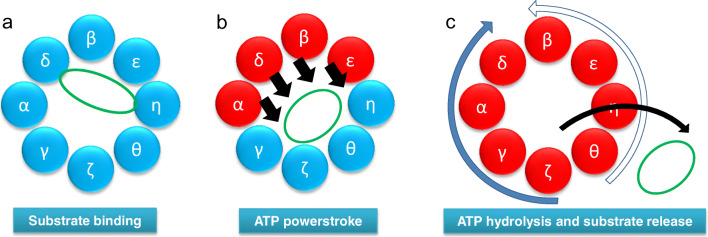
Fig. 2.
Model of the CCT folding cycle. a Actin (green) binds to the CCT oligomer in a 1.4 orientation. b ATP binding to high-affinity CCT subunits (red) leads to a powerstroke (black arrows) (Reissmann et al. 2012) and the actin molecule being released from one side of the chaperonin ring (Llorca et al. 2001). c After all CCT subunits have bound ATP, a sequential wave of ATP hydrolysis occurs either starting at CCTζ and proceeding clockwise around the ring (solid blue arrow, most probable) or starting at CCTθ and proceeding anti-clockwise around the ring (open blue arrow, less probable) (Gruber et al. 2017). Such a wave of ATP hydrolysis could be coupled to the ordered release of the folding substrate