Abstract
CRYAB is a small heat shock protein (sHSP) that has previously been shown to protect the heart against various cellular stresses; however, its precise function in myocardial cell injury caused by heat stress remains unclear. This study aimed to investigate the molecular mechanism by which CRYAB protects cardiomyocytes against heat stress. We constructed two H9C2 cell lines that stably express CRYAB protein to differing degrees: CRYAB-5 and CRYAB-7. Both CRYAB-5 and CRYAB-7 showed significantly reduced granular degeneration and vacuolar degeneration following heat stress compared to control cells. In addition, CRYAB overexpression in H9C2 cells relieved cell cycle proportion at the G0/G1 phase following heat stress compared to control cells. These protective effects were associated with the level of CRYAB protein expression. Our immunofluorescence analysis showed CRYAB could translocate from the cytoplasm to the nucleus under heat stress conditions, but that CRYAB co-localized with F-actin (which accumulates under stress conditions). Indeed, overexpression of CRYAB significantly reduced the aggregation of F-actin in H9C2 cells caused by heat stress. Furthermore, overexpressing CRYAB protein significantly reduced the apoptosis of cardiomyocytes induced by heat stress, likely by reducing the expression of cleaved-caspase 3. Collectively, our results show overexpression of CRYAB significantly increases the heat resistance of H9C2 cardiomyocytes, likely by reducing F-actin aggregation (thus stabilizing the cytoskeleton), regulating the cell cycle, and preventing caspase-mediated apoptosis.
Keywords: CRYAB, Cell cycle, F-actin, Apoptosis, Heat stress
Introduction
Heat stress occurs when the temperature exceeds the normal range and primarily affects the cardiovascular system. Heat stress could not only destroy the tight junctions between myocardial cells, but also could injure its structure and function (Cui and Sinoway 2014). Our previous study revealed necrosis in rat myocardium following heat stress for 40 min (Tang et al. 2016b). Such stress not only influences human health, but it can also lead to death in extreme cases (Crandall and Wilson 2015; Niu et al. 2009). For example, a heatwave claimed the lives of 15 people in Japan in 2014, with 8500 people hospitalized within a week (Crandall and Wilson 2015). In addition, heat stress can impact livestock productivity, with significant economic effects (Ahmad et al. 2017; Aitken-Buck and Lamberts 2017). Therefore, understanding the cellular response to heat stress is of utmost importance.
Heat stress is known to lead to oxidative stress and can cause a variety of proteins to aggregation, causing cytoskeletal and mitochondrial damage (Ahmad et al. 2017; Aitken-Buck and Lamberts 2017). To combat this damage and accelerate repair, heat shock proteins (HSPs) are expressed within mammalian cells, that bind to damaged proteins or peptide chains and help them refold into a functional protein (King and MacRae 2015). In addition to heat, HSPs are overexpressed in response to various other stresses, including bacteria, viruses, UV light, and cold temperatures (King and MacRae 2015). As well as refolding damaged or misfolded proteins, HSPs can stabilize newly formed proteins and protect them from unfolding (Sottile and Nadin 2018). They are also involved in a range of other cellular processes, such as apoptosis, the cell cycle, development, differentiation, and the immune response (Bakthisaran et al. 2015; Morrow et al. 2015).
HSPs are classified according to their molecular weight into several subfamilies, including the following: HSP110, HSP90, HSP70, HSP60, HSP40, small HSPs (sHSPs), and ubiquitin (King and MacRae 2015). sHSPs are important members of HSPs family. They not only act as molecular chaperone-like activities to prevent protein/peptide aggregation, but also participate in different cellular functions such as stress tolerance, maintenance of cytoskeletal integrity, apoptosis, signal transduction, cell cycle, and cell differentiation. In addition, sHSPs exhibit potent cardiac and neuroprotective effects by interacting with proteins to promote angiogenesis, or are anti-apoptotic and anti-inflammatory (Raman et al. 2015). αB-crystallin (CRYAB) is an important member of the sHSP subfamily, and its expression can be dramatically induced by stress and pathological conditions (Soti and Csermely 2000). While CRYAB also acts as a molecular chaperone, unlike true chaperones, it cannot renature proteins and release them; instead, CRYAB retains unfolded/misfolded proteins in large soluble aggregates (Yamamoto et al. 2014). CRYAB also has other known cellular functions, including reducing apoptosis, regulating oxidative stress and the cell cycle, and stabilizing the cytoskeleton (Arrigo 2012; Mercatelli et al. 2010).
Abundant expression of CRYAB has been detected in the eye lens, brain, heart, and skeletal muscle (Basha et al. 2012). CRYAB occupies 3–5% of total soluble protein in the heart and acts as an essential regulator to protect the heart against cellular stress (Dimauro et al. 2017). In addition, missense or deletion mutations within the CRYAB gene can cause restrictive, hypertrophic, and dilated cardiomyopathies (Muraleva et al. 2017). Moreover, CRYAB-KO mice have serious muscular dystrophy and can even undergo premature death (Brady et al. 2001). Indeed, CRYAB protects the heart against various cellular stresses, and that this protection is directly proportional to its upregulation and phosphorylation (Adhikari et al. 2011; Kamradt et al. 2002). For example, CRYAB overexpression in cultured cardiomyocytes and transgenic mouse hearts reduced ischemia/reperfusion injury and alleviated cardiac hypertrophy caused by pressure overload (Kumarapeli et al. 2008; Ray et al. 2001). Indeed, there is increasing interest in understanding how CRYAB acts as a regulator to contribute to cardiac cell survival.
In addition, upregulation or downregulation of sHSPs can produce beneficial results under different conditions. On the one hand, the upregulation of sHSPs provides beneficial effects in cardiac ischemia and neurodegenerative diseases such as amyotrophic lateral sclerosis (Tang et al. 2016a; Tang et al. 2016b). On the other hand, downregulation of sHSPs can provide beneficial results in cancer- and age-related macular degeneration (Muraleva et al. 2017). However, the precise function of CRYAB in myocardial cell injury caused by heat stress remains unclear.
Here, we tested the dynamic process of transcription and expression levels of CRYAB under heat stress and investigated whether overexpressing CRYAB in H9C2 cells would protect them against heat stress, and if so, determine its underlying mechanism of action.
Materials and methods
Cells and antibodies
H9C2 cells (American Type Culture Collection, ATCC), derived from embryonic rat hearts, were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 U penicillin and 100 μg/ml streptomycin (Gibco), and incubated in a 5% CO2 humidified atmosphere (Thermo, USA) at 37 °C. Cells were seeded in plastic tissue culture flasks (Corning, China) at a density of 1 × 105/mL and passaged every 48–72 h for maximum of 30 passages. Antibodies used in this study were the following: anti-CRYAB (ADI-SPA-222, ENZO, USA), anti-F-actin (NB100-64792), anti-GAPDH (ab181602, Abcam, USA), anti-cleaved-caspase 3 (9664S, CST, USA), HRP-conjugated goat anti-mouse IgG (BA1051), and HRP-conjugated goat anti-rabbit IgG (BA1054, Boster, China).
Transcription and expression levels of CRYAB in H9C2 cells under heat stress
H9C2 cells were seeded in a 60-mm dish and cultured at 37 °C for 36 h, then cells were transferred into a 5% CO2 humidified atmosphere at 42 °C for 0 h, 0.5 h, 2 h, and 3 h. Cells were harvested for RT-PCR and western blot analysis. The method were performed according to the literature (Yin et al. 2018).
Generation of recombinant plasmid and stable cell lines
To construct pcDNA3.1-CRYAB, the DNA fragment of CRYAB was amplified from the cDNA of H9C2 cells using the following primers: CRYAB-F (CGAGCTAGCATGGACATCGCC) and CRYAB-R (TACTTTGTTTAAACCTACTTCTTAGGGGCTGCGG). The amplified fragment was then inserted into the vector pcDNA3.1 ZEO+ (from our laboratory), which was linearized by digestion with NheI and PmeI (NEB, USA). To generate stably expressing H9C2 cell lines, we linearized the pcDNA3.1-CRYAB and pcDNA3.1 plasmids using ScaI (Takara, Japan), and then transfected them into H9C2 cells using the SuperFect Transfection Reagent (QIAGEN, USA). After incubating for 48 h, H9C2 cells were passaged into 6-well plates. When the cell confluence reached 40%, 300 μg/mL zeocine was added for 2 weeks, and the surviving single cell communities were passaged into 24-well plates using the clone ring. In this study, three cell strains were generated: one stably expressing the blank plasmid (control) and the other two stably overexpressing the CRYAB protein, albeit to varying degrees (CRYAB-5 and CRYAB-7). Three monoclonal cell lines were all passaged for maximum of 15 passages, that CRYAB-5 and CRYAB-7 were detected still to stably overexpress CRYAB protein in different degree.
Control, CRYAB-5, and CRYAB-7 cells (respectively) were seeded onto coverslips. When the cell confluence reached 80%, cells were exposed to 42 °C heat stress (for 0 h, 2 h, and 3 h) and immediately harvested (Xu et al. 2017; Xu et al. 2018) for histological, immunofluorescence, cell cycle, apoptosis, and western blot analysis (as detailed below).
Histological analysis
Briefly, cells were washed three times with phosphate-buffered saline (PBS), then fixed at room temperature with 4% paraformaldehyde for 30 min. Cells were then washed three times with PBS and stained with hematoxylin for 5 min. They were then washed with PBS (three times), counterstained under flowing water for 30 min, stained with eosin for 3 min, and then washed with PBS (three times). Cells were then dehydrated via an ethanol gradient of 75% ethanol, 85% ethanol, 95% ethanol (I), 95% ethanol (II), 100% ethanol (I), and 100% ethanol (II) (2 min each). Finally, the cells were made transparent via treatment with xylene (I) and xylene (II) for 2 min each, mounted with neutral resin, and observed under a light microscope using an Axio Imager.A2 instrument (Zeiss, Germany).
Immunofluorescence analysis of CRYAB and F-actin
Briefly, cells were washed three times with PBS, fixed at room temperature with 4% paraformaldehyde for 30 min, and washed again with PBS (three times). Cells were then permeabilized with 0.5% Triton × 100 for 20 min and washed with PBS (three times). After blocking with 5% bovine serum albumin (BSA) for 30 min, a 1:100 dilution of anti-CRYAB and anti-F-actin monoclonal antibodies were added and incubated overnight at 4 °C. Cells were then washed with PBS (three times) and a 1:100 dilution of FITC-conjugated goat anti-mouse IgG and TRITC-conjugated goat anti-rabbit IgM were added and incubated in the dark for 2 h at 37 °C. Finally, the cells were washed with PBS (three times), stained with DAPI for 2 min, washed with PBS (three times), and then mounted with 5 μL of anti-fluorescent quencher. The cellular distribution of CRYAB and F-actin were observed under an immunofluorescence microscope (Zeiss, Germany).
Cell cycle detection
We used the PI/RNase staining buffer (BD, USA) for cell cycle detection by flow cytometry, as per the manufacturer’s instructions. After heat stress, cells were digested with trypsin and collected and washed with PBS (twice). Then, 1 mL of pre-chilled 70% ethanol was added and gently pipetted to resuspend the cells. The cells were incubated at 4 °C overnight, and then collected by centrifugation at 1000 rpm for 5 min. The pelleted cells were washed with PBS (twice) and 500 μL PI/RNase staining buffer was added and gently pipetted to resuspend the cells. The cell samples were then incubated at room temperature for 15 min, and stored in a dark place at 4 °C before flow cytometry. Samples were tested within 1 h after staining, and the results were analyzed using FlowJo software.
Apoptosis detection
We used the Annexin V-FITC/PI staining buffer (Vazyme, China) for detecting apoptosis by flow cytometry, as per the manufacturer’s instructions. After heat stress, cells were digested with EDTA-free trypsin, centrifuged at 1000 rpm for 5 min, and washed three times with PBS. Then, 100 μL of 1 × binding buffer was added to resuspend the cells, and 5 μL each of Annexin V-FITC and PI was added for staining. After incubating in the dark at room temperature for 10 min, 400 μL of 1 × binding buffer was added and mixed before analysis by flow cytometry within 1 h. The results were analyzed using FlowJo software.
Western blot analysis
After heat stress, cells were washed with cold PBS (three times), and lysed in 100 μL RIPA lysis buffer (Dingguo Changsheng Biotechnology, China) containing 1% protease inhibitor (Nanjing Jiancheng Biochemical Reagent) on ice for 10 min. The supernatant was then collected and the protein concentration determined using the BCA Protein Assay Kit (Life Technologies, USA). SDS-loading buffer (Dingguo Changsheng Biotechnology, China) was added, and the samples were boiled for 15 min and stored at − 20 °C. Protein samples (containing 20 μg protein each) were separated by SDS-PAGE and transferred to polyvinylidene fluoride membrane (Bio-Rad, CA). After blocking with 5% non-fat milk in TBS-Tween 20 (TBST) buffer for 2 h, the membrane was incubated with the appropriate primary antibodies overnight (anti-CRYAB, 1:1000 dilution to make the final concentration of 2 μg/mL; anti-cleaved-caspase 3, 1:1000 dilution to make the final concentration of 80 ng/mL; anti-GAPDH, 1:10000 dilution to make the final concentration of 100 ng/mL), then washed with TBST buffer and incubated with the corresponding HRP-conjugated secondary antibodies (1:10000 dilution to make the final concentration of 100 ng/mL) for 2 h. After washing with TBST buffer, enhanced chemiluminescence detection regents (Thermo, USA) were added to the membranes and detected by the ImageQuant LAS4000 digital imaging system (GE Healthcare, Japan). The intensity of scanned bands was determined using Quantity One.
Statistical analysis
All experiments were performed in triplicate (n = 3), and the data were expressed as mean ± standard deviation (SD). Statistical significance was analyzed by one-way analysis of variance (ANOVA) with the least significant difference (LSD) multiple comparison test using the SPSS software v.20.0 (IBM, Armonk, NY) and assumed at P < 0.05 (* or #) or P < 0.01 (** or ##).
Results
Transcription level of CRYAB was increased while expression level was decreased under heat stress
Heat stress increased CRYAB transcript levels significantly at 2 h (~ 1.8-fold) and 3 h (~ 3.2-fold) (P < 0.01) compared to 0 h (Fig. 1). While the protein level of CRYAB after heat stress decreased significantly (P < 0.01) about 30% at 0.5 h and about 65% at 2 h and 3 h, compared to 0 h (Fig. 2).
Fig. 1.
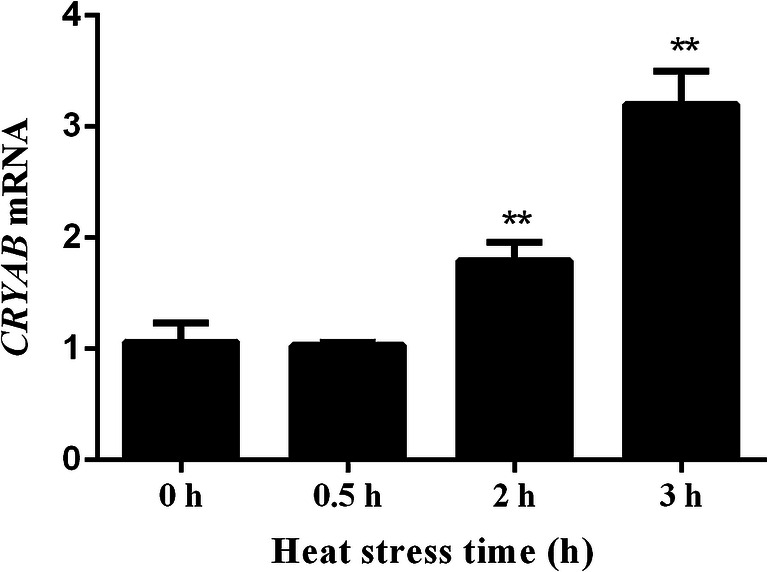
Transcription level of CRYAB relative to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Heat stress induced CRYAB transcription at 2 h and 3 h. *P < 0.05, **P < 0.01 compared with 0 h
Fig. 2.
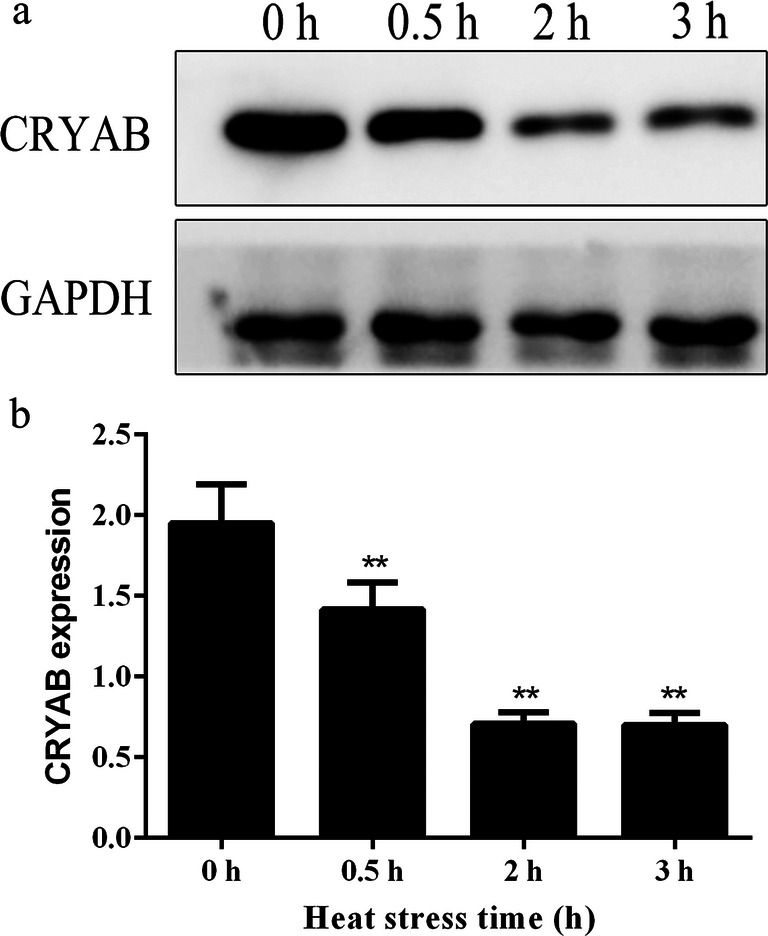
Expression level of CRYAB protein relative to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). a Western blot results. b Statistical analysis results. Heat stress decreased CRYAB protein level at 0.5 h, 2 h, and 3 h. *P < 0.05, **P < 0.01 compared with 0 h
Generation of CRYAB-overexpressing H9C2 cell lines
To construct pcDNA3.1-CRYAB, the coding sequence of CRYAB was amplified and identified by nucleic acid electrophoresis on a 1% agarose gel (Fig. 3a). The CRYAB fragment was then inserted into the pcDNA3.1 ZEO+ vector and verified by NheI and PmeI digestion (Fig. 3b). The recombinant plasmids were sequenced and showed 100% homology with the rat CRYAB gene sequence published in NCBI, indicating the CRYAB plasmids were successfully constructed.
Fig. 3.
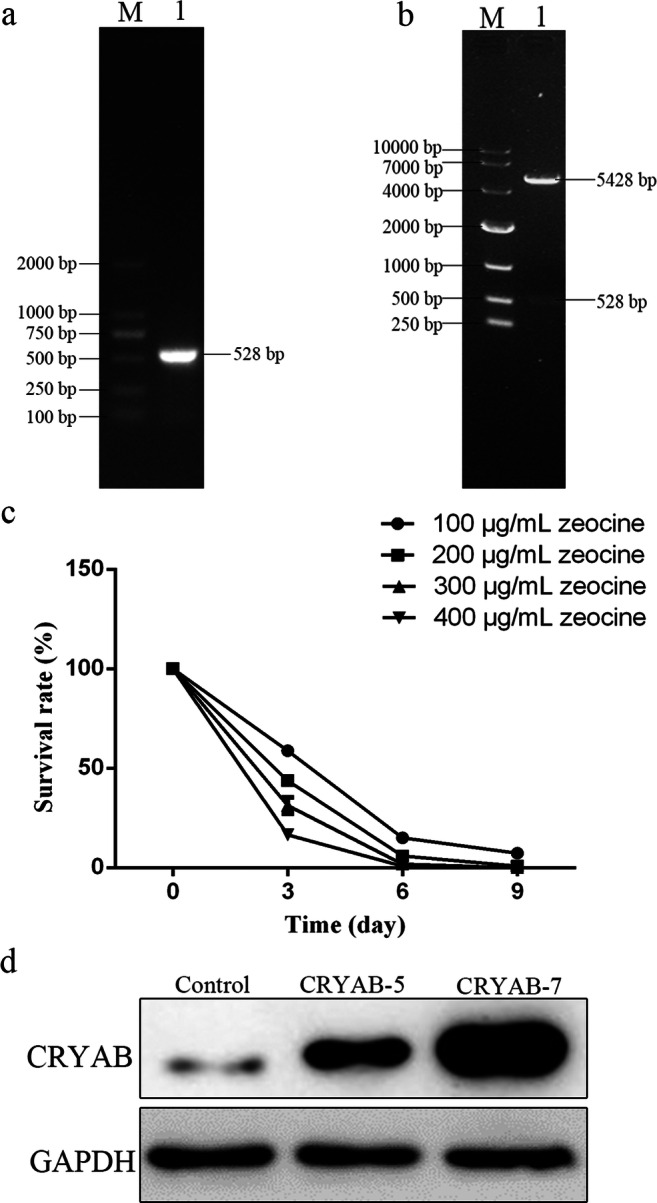
Generation of recombinant plasmid and stable cell lines. a PCR amplification of CRYAB produced a band of approximately 528 bp in size, consistent with the band of interest. b Double enzyme digestion of pcDNA3.1-CRYAB verifying insertion of the CRYAB fragment. c Killing effect of zeocine on H9C2 cells over time (300 μg/mL zeocine was selected for further screening). d Identification of stable cell lines by western blot. The CRYAB-5 and CRYAB-7 cell lines both overexpress CRYAB, albeit to differing degrees
Plasmids of pcDNA3.1-CRYAB and vector pcDNA3.1 were transfected into H9C2 cells and screened with 300 μg/mL zeocine (Fig. 3c) until monoclonal cell formation. In total, three monoclonal cell lines were generated: one contained the blank plasmid (control) and the other two contained CRYAB-overexpressing plasmids that expressed the CRYAB protein to differing degrees (CRYAB-5 and CRYAB-7; Fig. 3d).
CRYAB reduces damage of H9C2 cardiomyocytes caused by heat stress
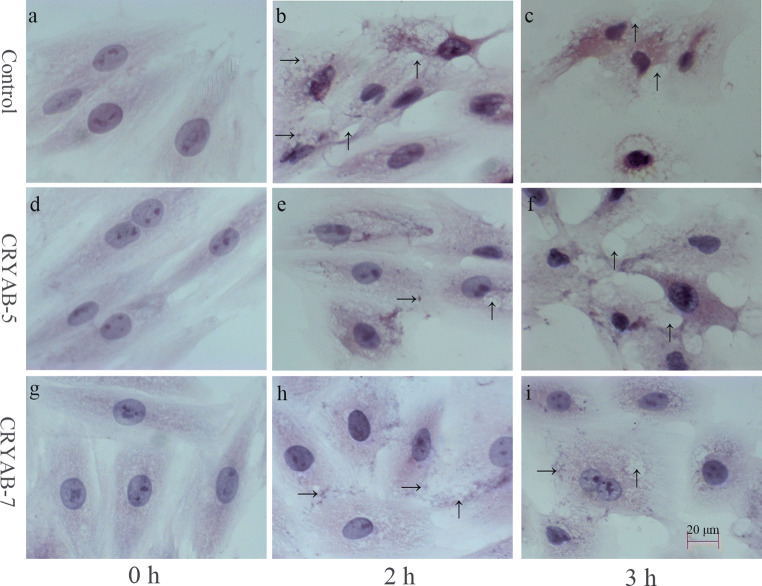
Ten fields in each of nine conditions were randomly selected for observation and photographing; the representative pathological changes are shown in Fig. 4. Overexpression of CRYAB protein did not affect the morphology of H9C2 cardiomyocytes under normal conditions, but it did significantly reduce the heat stress damage compared to controls when stimulated by the same degree of heat stress. Moreover, the cellular damage was further reduced with increasing CRYAB expression. In particular, granule denaturation and vacuolar degeneration appeared after 2 h of heat stress in control cells, and the phenomenon was more obvious when heat stress lasted for 3 h (i.e., the morphology changed from fusiform to polygonal, and the nuclei were deeply stained). Meanwhile, only slight granule denaturation and vacuolar degeneration were observed in the CRYAB-5 cell line, and no deep nuclei staining was observed. When the CRYAB-7 cell line was subjected to heat stress for 2 h and 3 h, the granular degeneration and vacuolar degeneration were all relatively mild, and the morphology changes observed in CRYAB-7 cells were apparently lower than those observed in the control and CRYAB-5 cells. Therefore, CRYAB overexpression reduces the morphological changes occurring in H9C2 cells caused by heat stress.
Fig. 4.
Hematoxylin and eosin staining of pathological lesions in control, CRYAB-5, and CRYAB-7 H9C2 cell lines following heat stress. a Control, heat 0 h; b: Control, heat 2 h, granular degeneration (→) and vacuolar degeneration (↑); c: Control, heat 3 h, vacuolar degeneration (↑); d: CRYAB-5, heat 0 h; e: CRYAB-5, heat 2 h, granular degeneration (→) and vacuolar degeneration (↑); f: CRYAB-5, heat 3 h, vacuolar degeneration (↑); g: CRYAB-7, heat 0 h; h: CRYAB-7, heat 2 h, granular degeneration (→) and vacuolar degeneration (↑); i: CRYAB-7, heat 3 h, granular degeneration (→) and vacuolar degeneration (↑)
CRYAB reduces the aggregation of F-actin in H9C2 cardiomyocytes
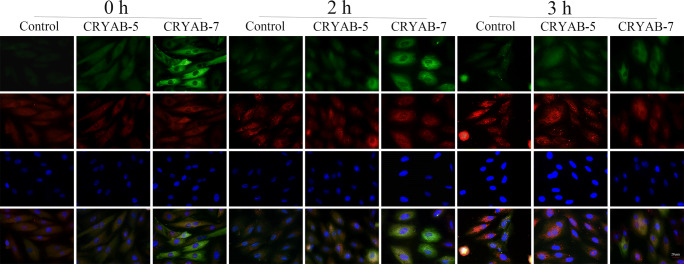
Co-localization of CRYAB and F-actin was detected by immunofluorescence, ten fields in each of the nine conditions were randomly selected for observation and photographing; the representative fluorescence results were shown in Fig. 5. F-actin (red signal) was evenly distributed in the cytoplasm under normal conditions; however, F-actin accumulated to form red punctate signals when the cells were stimulated by heat stress (2 h). These punctate signals became more evident with the prolongation of heat stress (3 h). Meanwhile, overexpression of CRYAB (green signal) significantly decreased the aggregation of F-actin. The effect of CRYAB on F-actin aggregation was dependent on the expression level: CRYAB-5 cells still showed some red punctate signals after 3 h of heat stress while the CRYAB-7 cell line had no obvious red punctate signals (Fig. 5). Together, these results indicate CRYAB overexpression reduces the aggregation of F-actin in H9C2 cardiomyocytes.
Fig. 5.
Immunofluorescence staining showing the co-localization of F-actin and CRYAB in control, CRYAB-5, and CRYAB-7 H9C2 cell lines following heat stress. F-actin shown in red, CRYAB shown in green, and DAPI shown in blue
CRYAB relieves cell cycle proportion at the G0/G1 stage
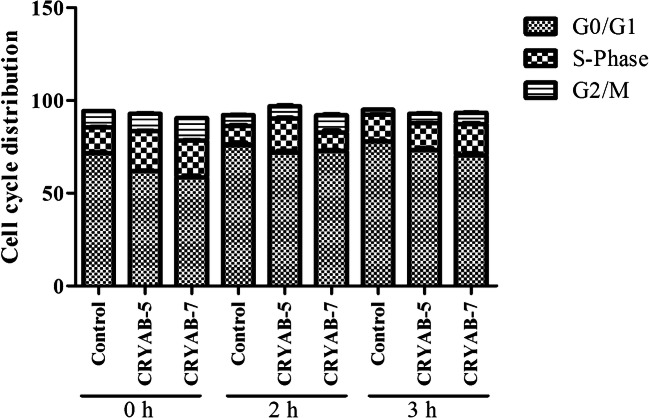
Cells may become arrested in the G0/G1 phase after heat stress to prevent cell proliferation. Thus, we detected the proportion of cells at each stage of the cell cycle by flow cytometry (Fig. 6) and the statistical analysis results are shown in Table 1. Under normal conditions (0 h), overexpression of CRYAB significantly reduced the proportion of cells in the G0/G1 phase, but significantly increased the proportion of cells in the S and G2/M phase compared to the control, and the effect was elevated with increasing CRYAB overexpression. The proportion of cells in G0/G1 phase increased in all three cell lines after 2 and 3 h of heat stress compared to 0 h. At 3 h after heat stress, the proportion of cells in S phase was similar in control cells and CRYAB-5 cells but was significantly greater for CRYAB-7 cells. Also at 3 h after heat stress, compared with the control cells, the proportion of CRYAB-5 and CRYAB-7 cells in the G0/G1 phase was significantly lower, while in the G2/M phase, the proportion of CRYAB cells was significantly higher than the control cells.
Fig. 6.
Cell cycle analysis in control, CRYAB-5, and CRYAB-7 H9C2 cell lines following heat stress
Table 1.
Statistical analysis of cell cycle distribution
| G0/G1 | S-Phase | G2/M | ||
|---|---|---|---|---|
| 0 h | Control | 71.41 ± 0.84 | 14.19 ± 0.19 | 8.59 ± 0.13 |
| CRYAB-5 | 61.96 ± 0.70 ** ## | 21.47 ± 0.36 ** ## | 9.44 ± 0.37 * # | |
| CRYAB-7 | 58.47 ± 1.40 ** ## | 20.28 ± 0.44 ** ## | 11.69 ± 0.83 ** ## | |
| 2 h | Control | 75.76 ± 2.74 * | 10.52 ± 0.68 ** | 5.75 ± 0.17 ** |
| CRYAB-5 | 71.75 ± 1.84 # | 18.37 ± 0.97 ** ## | 6.75 ± 0.51 ** # | |
| CRYAB-7 | 67.73 ± 3.54 * ## | 11.16 ± 0.98 ** | 9.44 ± 0.77 * ## | |
| 3 h | Control | 77.79 ± 1.17 ** | 14.51 ± 0.31 | 2.58 ± 0.02 ** |
| CRYAB-5 | 73.36 ± 1.93 # | 14.31 ± 0.60 | 5.18 ± 0.12 ** ## | |
| CRYAB-7 | 70.30 ± 2.07 ## | 17.03 ± 0.76 ** ## | 5.98 ± 0.21 ** ## |
*P < 0.05, **P < 0.01 compared with 0 h in the control group; #P < 0.05, ##P < 0.01 indicates CRYAB-5 and CRYAB-7 groups compared with the control group at the same timepoint
CRYAB reduces caspase-mediated apoptosis of H9C2 cardiomyocytes
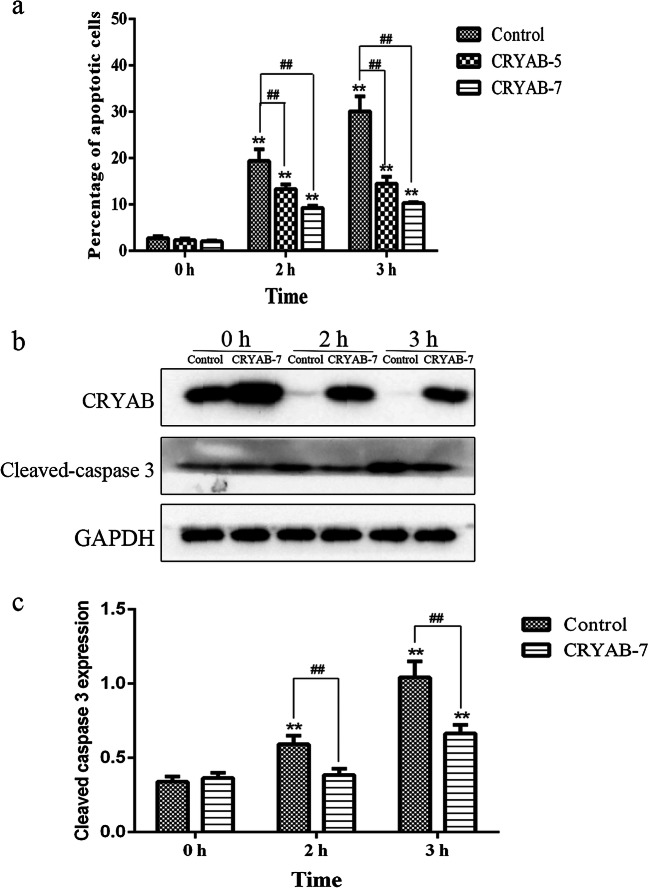
Next, we detected the percentage of cells undergoing apoptosis by flow cytometry (Fig. 7a). Overexpression of CRYAB significantly reduced cellular apoptosis caused by heat stress. When the control cell line was treated with heat stress for 2 h, the apoptotic rate was about 20%; this apoptotic rate increased to 30% after 3 h of heat stress. Meanwhile, the apoptotic rate in the CRYAB-5 cell line was about 13% after 2 h of heat stress, and about 15% after 3 h of heat stress. The apoptotic rate was less than 10% when CRYAB-7 cells were subjected to heat stress for 2 h, and was only about 10% after 3 h.
Fig. 7.
Apoptosis detection a in control, CRYAB-5, and CRYAB-7 H9C2 cell lines following heat stress. *P < 0.05, **P < 0.01 compared with 0 h in the control group; #P < 0.05, ##P < 0.01 indicates CRYAB-5 and CRYAB-7 groups compared with the control group at the same timepoint. b, c Western blot analysis showing the expression levels of CRYAB protein and cleaved-caspase 3 protein in control and CRYAB-7 cells following heat stress
To explore the mechanism of the anti-apoptotic effect of CRYAB overexpression, we examined the protein levels of both CRYAB and cleaved-caspase 3 in control and CRYAB-7 cells via western blot (Fig. 7b). The CRYAB-7 cell line maintained expression of the CRYAB protein under both normal and heat stress conditions at 2 h and 3 h, although CRYAB expression did decrease slightly after heat stress. While the protein level of cleaved-caspase 3 increased in the CRYAB-7 cell line with prolonged heat stress, cleaved-caspase 3 levels were significantly lower in the CRYAB-7 cells than in the control cells. Therefore, CRYAB reduces the apoptosis of H9C2 cardiomyocytes, likely by downregulating cleaved-caspase 3.
Discussion
Overall, we found the transcription and expression level of CRYAB in H9C2 cells was associated with increased heat resistance, but these changes were in opposite directions. Heat stress increased CRYAB transcript level about 1.8-fold at 2 h and about 3.2-fold at 3 h, while, the CRYAB protein level decreased about 30% at 0.5 h and about 65% at 2 h and 3 h. This result was consistent with previously observed decrease in CRYAB in rat heart following heat stress for 20, 60, 80, and 100 min (Tang et al. 2016a). We speculated that the decrease of CRYAB protein might be due to CRYAB being phosphorylated when it functions, that is easily recognized and degraded by the proteasome in H9C2 cardiomyocytes under heat stress conditions. On the other hand, the apparent decrease of CRYAB protein might be because the antibody epitope is masked by its interaction with another protein (Ito et al. 1997; Kato et al. 1998; Ito et al. 2001). The underlying reasons clearly require further study. CRYAB was previously shown to play important roles in protecting cytoskeletal integrity and in regulating apoptosis and the cell cycle (Arrigo 2012; Mercatelli et al. 2010; Zhang et al. 2015). We found H9C2 cells overexpressing CRYAB exhibited less intracellular degeneration than control H9C2 cells subjected to the same heat stress stimulus. This indicates that the CRYAB protein plays an important protective role in H9C2 cells against heat stress injury. Our findings are consistent with a previous study showing that aspirin protects chicken cardiomyocytes from heat stress injury by upregulating expression of the CRYAB protein (Tang et al. 2016b).
CRYAB can also prevent diseases such as stroke by maintaining the cytoskeleton. CRYAB is localized in the I-band and M-line regions of myofibrils and stabilizes the myofibrils in cardiomyocytes in vitro (Laplante et al. 1998). Under stress conditions, CRYAB binds to Hsp27, relocates to the cytoskeleton, and binds to microtubules through microtubule-associated proteins to protect cells from damage (Martin et al. 2001). F-actin is a cytoskeletal protein that plays an important role in maintaining and stabilizing the cytoskeletal structure (David et al. 2008). We found that F-actin was filamentous and distributed evenly in the cytoplasm under normal conditions, but accumulated upon heat stress. We also observed co-localized expression of CRYAB and F-actin, and that overexpression of CRYAB reduced the aggregation of F-actin. Our results suggest that CRYAB may protect H9C2 cells from heat injury through combination with F-actin, thus preventing F-actin aggregation and stabilizing the cytoskeleton. This viewpoint is supported by a number of prior research results (Salinthone et al. 2008).
The expression levels of αA- and αB-crystallin differ during the cell cycle in lens epithelial cells, and knockout or overexpression of these proteins affects cell cycle progression (Andley et al. 2001; Bai et al. 2004). Cell cycle has a tightly regulated sequence, consist of G0/G1 phase, S phase, G2 phase, and M phase. However, when cells suffered damage, the cell cycle appeared arrest and was prolonged, that provided extra time for cells to repair the damage and was beneficial for maintenance of gene stability (Canaud and Bonventre 2015). In our study, overexpression of CRYAB under normal conditions allowed H9C2 cells to enter the cell division phase by reducing the proportion of cells in the G0/G1 phase. This indicated that overexpression of CRYAB could shorten cell cycle and promote cell proliferation, and is consisted with the literature where si-CRYAB cells appeared prolonged in the G1 phase (Shi et al. 2017). In addition, CRYAB overexpression significantly alleviated the proportion of cells at the G0/G1 phase following heat stress.
sHSPs also play important anti-apoptotic roles, especially Hsp27 and CRYAB (Arrigo 2012; Mercatelli et al. 2010; Paul et al. 2002). CRYAB reduces the damage induced by oxidative stress and decreases apoptosis induced by TNF-α and drugs such as staurosporine and doxorubicin (Arrigo 2012; Mercatelli et al. 2010). Our study also showed the anti-apoptotic effect of CRYAB overexpression when H9C2 cells were exposed to heat stress. Furthermore, our results indicate that the anti-apoptotic effect of CRYAB might be related to reduce cleaved-caspase 3 levels. Indeed, a growing number of studies have found that increasing the expression level of CRYAB can enhance the anti-stress tolerance of cells (Kumarapeli et al. 2008; Ray et al. 2001).
Conclusion
In summary, overexpression of CRYAB protein significantly increases the heat resistance of H9C2 cardiomyocytes by binding to F-actin, regulating the cell cycle, and reducing caspase-mediated apoptosis; however, the underlying mechanism(s) by which CRYAB exerts these protective responses in cardiomyocytes following heat stress requires further investigation.
Funding
The current study was supported by the grants from the National Natural Science Foundation of China (grant no. 31672520), the Fundamental Research Funds for the Central Universities (grant no. KJQN201709), the National Natural Science Foundation of China (grant no. 31602027), the National Natural Science Foundation of China (grant no. 31372403), Jiangsu Natural Science Foundation of China (grant no. BK20160732), China Postdoctoral Science Foundation (2016M591860), and Shandong Natural Science Foundation of China (grant no. ZR2016CM40).
Footnotes
Bin Yin and Shu Tang contributed equally to this work.
References
- Adhikari AS, Singh BN, Rao KS, Rao CM. AlphaB-crystallin, a small heat shock protein, modulates NF-kappaB activity in a phosphorylation-dependent manner and protects muscle myoblasts from TNF-alpha induced cytotoxicity. Biochim Biophys Acta. 2011;1813:1532–1542. doi: 10.1016/j.bbamcr.2011.04.009. [DOI] [PubMed] [Google Scholar]
- Ahmad G, Agarwal A, Esteves SC, Sharma R, Almasry M, Al-Gonaim A, AlHayaza G, Singh N, Al-Kattan L, Sannaa WM, Sabanegh E. Ascorbic acid reduces redox potential in human spermatozoa subjected to heat-induced oxidative stress. Andrologia. 2017;49:e12773. doi: 10.1111/and.12773. [DOI] [PubMed] [Google Scholar]
- Aitken-Buck HM, Lamberts RR. To the heart of activation heat. J Physiol. 2017;595:4577–4578. doi: 10.1113/JP274582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andley UP, Song Z, Wawrousek EF, Brady JP, Bassnett S, Fleming TP. Lens epithelial cells derived from aB-crystallin knockout mice demonstrate hyperproliferation and genomic instability. FASEB J. 2001;15:221–229. doi: 10.1096/fj.00-0296com. [DOI] [PubMed] [Google Scholar]
- Arrigo AP. Pathology-dependent effects linked to small heat shock proteins expression: an update. Scientifica. 2012;2012:1–19. doi: 10.6064/2012/185641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F, Xi J, Higashikubo R, Andley UP. A comparative analysis of alphaA- and alphaB-crystallin expression during the cell cycle in primary mouse lens epithelial cultures. Exp Eye Res. 2004;79:795–805. doi: 10.1016/j.exer.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Bakthisaran R, Tangirala R, Rao CM. Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854:291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Basha E, O’Neill H, Vierling E. Small heat shock proteins and alpha-crystallins: dynamic proteins with flexible functions. Trends Biochem Sci. 2012;37:106–117. doi: 10.1016/j.tibs.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. aB-crystallin in lens development and muscle integrity: a gene knockout approach. Investig Ophthalmol Vis Sci. 2001;42:2924–2934. [PubMed] [Google Scholar]
- Crandall CG, Wilson TE. Human cardiovascular responses to passive heat stress. Comprehensive Physiology. 2015;5:17–43. doi: 10.1002/cphy.c140015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaud G, Bonventre J. Cell cycle arrest and the evolution of chronic kidney disease from acute kidney injury. Nephrol Dial Transplant. 2015;30:575–583. doi: 10.1093/ndt/gfu230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Sinoway LI. Cardiovascular responses to heat stress in chronic heart failure. Current Heart Failure Reports. 2014;11:139–145. doi: 10.1007/s11897-014-0191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David AM, Elisa KT, Mark R, Roger S, George Y, Kenneth K, Chan EC. Contraction in human myometrium is associated with changes in small heat shock proteins. Endocrinology. 2008;149:245–252. doi: 10.1210/en.2007-0662. [DOI] [PubMed] [Google Scholar]
- Dimauro I, Antonioni A, Mercatelli N, Caporossi D (2017) The role of alphaB-crystallin in skeletal and cardiac muscle tissues. Cell Stress Chaperones. 10.1007/s12192-017-0866-x. [DOI] [PMC free article] [PubMed]
- Ito H, Kamei K, Iwamoto I. Phosphorylation induced change of the oligomerization state of alpha B-crystallin. J Biol Chem. 2001;276:5346–5352. doi: 10.1074/jbc.M009004200. [DOI] [PubMed] [Google Scholar]
- Ito H, Okamoto K, Nakayama H. Phosphorylation of alphaB-crystallin in response to various types of stress. J Biol Chem. 1997;272:29934–29941. doi: 10.1074/jbc.272.47.29934. [DOI] [PubMed] [Google Scholar]
- Kamradt MC, Chen F, Sam S, Cryns VL. The small heat shock protein alpha B-crystallin negatively regulates apoptosis during myogenic differentiation by inhibiting caspase-3 activation. J Biol Chem. 2002;277:38731–38736. doi: 10.1074/jbc.M201770200. [DOI] [PubMed] [Google Scholar]
- Kato K, Ito H, Kamei K. Phosphorylation of alphaB-crystallin in mitotic cells and identification of enzymatic activities responsible for phosphorylation. J Biol Chem. 1998;273:28346–28354. doi: 10.1074/jbc.273.43.28346. [DOI] [PubMed] [Google Scholar]
- King AM, Macrae TH. Insect heat shock proteins during stress and diapause. Annu Rev Entomol. 2015;60:59–75. doi: 10.1146/annurev-ento-011613-162107. [DOI] [PubMed] [Google Scholar]
- Kumarapeli AR, Su H, Huang W, Tang M, Zheng H, Horak KM, Li M, Wang X. Alpha B-crystallin suppresses pressure overload cardiac hypertrophy. Circ Res. 2008;103:1473–1482. doi: 10.1161/CIRCRESAHA.108.180117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante AF, Moulin V, Auger FA, Landry J, Li H, Morrow G, Tanguay RM, Germain L. Expression of heat shock proteins in mouse skin during wound healing. J Histochem Cytochem. 1998;46:1291–1301. doi: 10.1177/002215549804601109. [DOI] [PubMed] [Google Scholar]
- Martin W, Rainer B, Joachim B, Rudolf D, Gerlinde G, Heinz B, Lutsch G. Defined sequence segments of the small heat shock proteins HSP25 and aB-crystallin inhibit actin polymerization. Eur J Biochem. 2001;268:2083–2090. doi: 10.1046/j.0014-2956.2001.02506.x. [DOI] [PubMed] [Google Scholar]
- Mercatelli N, Dimauro I, Ciafre SA, Farace MG, Caporossi D. AlphaB-crystallin is involved in oxidative stress protection determined by VEGF in skeletal myoblasts. Free Radic Biol Med. 2010;49:374–382. doi: 10.1016/j.freeradbiomed.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Morrow G, Hightower LE, Tanguay RM. Small heat shock proteins: big folding machines. Cell Stress Chaperones. 2015;20:207–212. doi: 10.1007/s12192-014-0561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muraleva NA, Devyatkin VA, Kolosova NG. Phosphorylation of alphaB-crystallin in the myocardium: analysis of relations with aging and cardiomyopathy. Exp Gerontol. 2017;95:26–33. doi: 10.1016/j.exger.2017.05.009. [DOI] [PubMed] [Google Scholar]
- Niu ZY, Liu FZ, Yan QL, Li WC. Effects of different levels of vitamin E on growth performance and immune responses of broilers under heat stress. Poult Sci. 2009;88:2101–2107. doi: 10.3382/ps.2009-00220. [DOI] [PubMed] [Google Scholar]
- Paul C, Manero F, Gonin S, Kretz-Remy C, Virot S, Arrigo AP. Hsp27 as a negative regulator of cytochrome c release. Mol Cell Biol. 2002;22:816–834. doi: 10.1128/MCB.22.3.816-834.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman B, Ramakrishna T, Mohan CR. Small heat shock proteins: role in cellular functions and pathology. Biochim Biophys Acta. 2015;1854:291–319. doi: 10.1016/j.bbapap.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Ray PS, Martin JL, Swanson EA, Otani H, Dillmann WH, Das DK. Transgene overexpression of aB crystallin confers simultaneous protection against cardiomyocyte apoptosis and necrosis during myocardial ischemia and reperfusion. FASEB J. 2001;15:393–402. doi: 10.1096/fj.00-0199com. [DOI] [PubMed] [Google Scholar]
- Salinthone S, Tyagi M, Gerthoffer WT. Small heat shock proteins in smooth muscle. Pharmacol Ther. 2008;119:44–54. doi: 10.1016/j.pharmthera.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Yang X, Bu X, Hou N, Chen P. Alpha B-crystallin promotes the invasion and metastasis of colorectal cancer via epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2017;489:369–374. doi: 10.1016/j.bbrc.2017.05.070. [DOI] [PubMed] [Google Scholar]
- Soti C, Csermely P. Molecular chaperones and the aging process. Biogerontology. 2000;1:225–233. doi: 10.1023/A:1010082129022. [DOI] [PubMed] [Google Scholar]
- Sottile ML, Nadin SB. Heat shock proteins and DNA repair mechanisms: an updated overview. Cell Stress Chaperones. 2018;23:303–315. doi: 10.1007/s12192-017-0843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang S, Chen H, Cheng Y, Nasir MA, Kemper N, Bao E. Expression profiles of heat shock protein 27 and alphaB-crystallin and their effects on heat-stressed rat myocardial cells in vitro and in vivo. Mol Med Rep. 2016;13:1633–1638. doi: 10.3892/mmr.2015.4693. [DOI] [PubMed] [Google Scholar]
- Tang S, Yin B, Song E, Chen H, Cheng Y, Zhang X, Bao E, Hartung J (2016b) Aspirin upregulates alphaB-Crystallin to protect the myocardium against heat stress in broiler chickens. Sci Rep 6. 10.1038/srep37273 [DOI] [PMC free article] [PubMed]
- Xu J, Tang S, Yin B, Sun J, Song E, Bao E. Co-enzyme Q10 and acetyl salicylic acid enhance Hsp70 expression in primary chicken myocardial cells to protect the cells during heat stress. Mol Cell Biochem. 2017;435:73–86. doi: 10.1007/s11010-017-3058-1. [DOI] [PubMed] [Google Scholar]
- Xu J, Tang S, Yin B, Sun J, Bao E (2018) Co-enzyme Q10 upregulates Hsp70 and protects chicken primary myocardial cells under in vitro heat stress via PKC/MAPK. Mol Cell Biochem. 10.1007/s11010-018-3356-2 [DOI] [PubMed]
- Yamamoto S, Yamashita A, Arakaki N, Nemoto H, Yamazaki T. Prevention of aberrant protein aggregation by anchoring the molecular chaperone alphaB-crystallin to the endoplasmic reticulum. Biochem Biophys Res Commun. 2014;455:241–245. doi: 10.1016/j.bbrc.2014.10.151. [DOI] [PubMed] [Google Scholar]
- Yin B, Tang S, Sun J, Zhang X, Xu J, Di L, Li Z, Hu Y, Bao E. Vitamin C and sodium bicarbonate enhance the antioxidant ability of H9C2 cells and induce HSPs to relieve heat stress. Cell Stress Chaperones. 2018;23:735–748. doi: 10.1007/s12192-018-0885-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Ezemaduka AN, Wang Z, Hu H, Shi X, Liu C, Lu X, Fu X, Chang Z, Yin CC (2015) A novel mechanism for small heat shock proteins to function as molecular chaperones. Sci Rep 5. 10.1038/srep08811 [DOI] [PMC free article] [PubMed]