Figure 3.
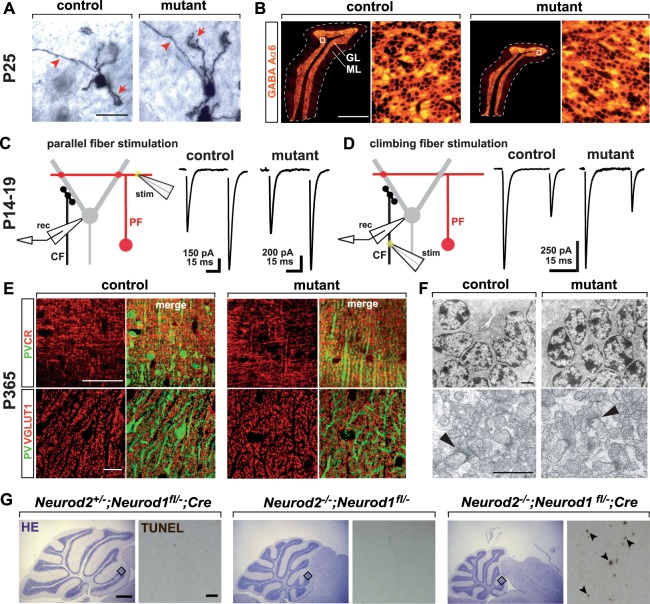
Surviving NeuroD2-deficient granule cells terminally differentiate and functionally integrate into excitatory cerebellar circuits. (A) Granule cells in Neurod2−/− mutants extend claw-like telodendria (arrow) and axons projecting towards the ML (arrowhead). Golgi impregnation of sagittal sections (P25). Scale bar, 20 µm. (B) Fluorescent immunostaining for GABA Aα6 receptor on sagittal sections (lobule 5) from Neurod2−/− mutants and controls (P25). (Right panels) magnification of boxed area in left panels. Scale bar, 600 µm. (C) Left: Schematic of PF EPSC recordings from Purkinje cells. PFs were stimulated in the ML (stim) while Purkinje cells were held in whole-cell voltage clamp (rec). Right: Representative traces of PF EPSCs from controls and Neurod2−/− mutants. Paired-pulses reveal similar EPSC2 facilitation compared to EPSC1. (D) Left: Schematic of recordings of CF EPSCs from Purkinje cells. CFs were stimulated in the GL (stim) while Purkinje cells were held in whole-cell voltage clamp (rec). Right: Representative traces of CF EPSCs from control and Neurod2−/− mutants. Paired-pulses reveal similar depression of EPSC2 compared to EPSC1. (E) Normal number and distribution of PFs and their terminals in Neurod2−/− mutants compared to controls. Fluorescent immunostaining for Purkinje cell dendrites (PV) and PFs (calretinin, CR; or VGLUT1) (age 1 year). Note differences in PV staining due to loss of PV+ MLIs in Neurod2−/− mutants. Scale bars, 20 µm. (F) (Upper panels) Electron microscopy of granule cells in Neurod2−/− mutants exhibits a similar distribution of nuclear heterochromatin compared to controls (age 1 year). Scale bar, 1.7 µm. (Lower panels) Ultrastructure of PF/Purkinje cell synapses (arrowheads) in Neurod2−/− mutants compared to controls (age 1 year). Scale bar, 1 µm. (G) Overlapping NeuroD1 and NeuroD2 functions in postmigratory granule cell survival. (left) Normal cerebellar size and absence of granule cell apoptosis (TUNEL assay of GL in lobule 2) at P21 in conditional Neurod1 null mutants (NeuroD1 deficiency in postmigratory granule cells) with one wildtype copy of Neurod2 (Neurod2+/−; Neurod1fl/−; Cre). (Middle) Neurod2−/− mutants with one functional copy of Neurod1 (Neurod2−/−; Neurod1fl/−) display reduced cerebellar size similar to Neurod2−/− single mutants (see B). Note that granule cell apoptosis has ceased by P21. (right) Reduced cerebellar size and granule cell apoptosis (arrowheads) in Neurod1/2 double mutants (Neurod2−/−; Neurod1fl/−; Cre). Scale bars, 500 µm (H&E stainings); 30 µm (TUNEL).