Abstract
Background
Treatment of hepatic hydrothorax (HH) generally involves sodium restriction, diuretics, and serial thoracentesis. In more advanced cases, transjugular intrahepatic portosystemic shunt and liver transplantation may be required. Previously, indwelling tube drainage has been avoided due to concerns regarding high complication rates and overall poor outcomes. Recently, indwelling pleural catheters (IPCs) have been proposed as a novel treatment option for HH.
Methods
This study was a retrospective review of patients who had undergone IPC placement for HH over a 10-year period at a large liver transplant referral center. We tracked outcomes, including complication rates and liver transplantation, as well as biomarkers of nutritional status.
Results
Sixty-two patients underwent IPC placement between 2007 and 2017, with 33 IPCs (53%) placed as a bridge to liver transplantation. Complications were recorded in 22 patients (36%); empyema was the most common, diagnosed in 10 patients (16.1%). Ten patients evaluated for liver transplantation underwent successful transplantation following IPC placement. There were statistically significant decreases in both BMI and serum albumin levels following IPC placement.
Conclusions
IPCs represent a potential treatment for refractory HH and should be used with caution in patients eligible for liver transplantation. Ideally, IPC use for these patients would be evaluated by a multidisciplinary team. IPC use may lead to small decreases in BMI and serum albumin levels in patients over time.
Key Words: empyema, hepatic hydrothorax, indwelling pleural catheter, liver transplant
Abbreviations: HH, hepatic hydrothorax; IPC, indwelling pleural catheter; MELD-Na, Model for End-Stage Liver Disease–Sodium; TIPS, transjugular intrahepatic portosystemic shunt
FOR EDITORIAL COMMENT, SEE PAGE 251
Hepatic hydrothorax (HH) is a pleural effusion in a patient with portal hypertension with no primary cardiac, pulmonary, or pleural disease.1 It is an uncommon complication of portal hypertension, occurring in approximately 5% to 10% of patients with cirrhosis.1, 2, 3, 4 Development of HH carries a poor prognosis, with a median survival of 8.6 months.5
Management of HH focuses on salt restriction, diuretics, and serial thoracentesis.2 Transjugular intrahepatic portosystemic shunt (TIPS) can be an effective treatment for those who fail to show improvement with medical management, with success rates as high as 80%.6, 7 Unfortunately, many patients with HH have contraindications to TIPS (ie, hepatic encephalopathy, hyperbilirubinemia). Transplantation is the only other treatment shown to be effective for patients who fail to improve with medical management.8
Historically, tube thoracostomy has been strongly discouraged in HH because of concerns regarding complications, including renal failure, infection, electrolyte depletion, and protein loss.9, 10, 11 However, newer indwelling tunneled pleural catheters (IPCs) have been proposed as a novel treatment approach for the patient with refractory hydrothorax. The tunneled nature of these catheters theoretically ameliorates the risk of infection. These pleural catheters are widely accepted as an option for symptomatic management of malignant pleural effusions,12, 13 with increasing attention to their potential therapeutic applications in nonmalignant effusions.14, 15 Some centers have begun using IPCs for treatment of HH.16, 17 Recently, a pilot study with 24 transplant-eligible patients showed the feasibility of this approach.18
The purpose of the present study was to assess outcomes and complication rates of patients who underwent IPC placement for HH at a large tertiary referral center for patients with advanced liver disease. To our knowledge, our study, which includes a significant portion of patients who were eligible for liver transplantation, represents the largest series of such patients described in the literature to date.
Patients and Methods
A single-center, retrospective analysis was performed on all patients from 2007 to 2017 with a diagnosis of cirrhosis who underwent placement of an IPC. Data were extracted from the electronic medical record system (Cerner Corporation) using the search terms “cirrhosis” and “IR Insert Tunneled Cath Pleural” to capture those IPCs placed by the interventional radiology service at our institution. A subsequent query using the terms “cirrhosis” and “pleural catheter” was performed under the endoscopy schedule in the same electronic records system to identify those patients who underwent IPC placement by the pulmonary service. At our institution, it is standard that interventional radiology places either the Aspira (C.R. Bard) or PleurX (BD) catheters, whereas the pulmonary service exclusively places PleurX catheters.
Inclusion criteria included age ≥ 18 years, diagnosis of cirrhosis and HH, and IPC placement for management of recurrent effusion. Exclusion criteria included patients with a pleural effusion due to a condition other than HH or those who had a diagnosis of empyema prior to IPC placement. A diagnosis of HH was confirmed by the presence of a recurrent effusion in the setting of cirrhosis for which alternate etiologies had been excluded. Results of previous pleural fluid studies were examined when possible. The institutional review board of Indiana University School of Medicine approved the study protocol (protocol number 1701955275).
Procedure
Patients underwent IPC placement per standard practice of the interventional radiology service or the pulmonary service. Both the PleurX and Aspira catheters are 15.5F silicone tubes designed for placement using local anesthetic and light to moderate sedation.19 Procedures were performed during either inpatient or outpatient encounters. All procedures were performed in operating rooms using standard sterile procedures. Peri-procedural antibiotics were administered in some cases. Patients were provided the appropriate drainage equipment per standard practice, and follow-up was dictated by the patient’s clinical course.
Data Collection
Data collected at the time of IPC placement included age, sex, BMI, etiology of cirrhosis, serum bilirubin, creatinine, international normalized ratio, sodium, and albumin levels. In cases in which laboratory and BMI data were not available on the day of IPC placement, values within 14 days prior to the procedure were accepted. Additional baseline data points included previous therapeutic interventions for hydrothorax (including salt restriction, diuretics, previous thoracentesis, previous TIPS, pleurodesis, or octreotide). Date, laterality, type of IPC, reason for IPC placement, and liver transplant status were also collected at baseline. Patients who were listed for transplant, or actively under evaluation for listing, were classified as “bridge to transplant,” whereas those who were definitively not transplant candidates, including those enrolling in comfort-based care, were classified as “palliative.” There were some patients for whom it was not clear from available documentation whether the IPC was placed with palliative intent or as a bridge to transplant; these patients were classified as “unclear.”
Follow-up data included presence of complications, categorized as empyema, catheter site infection, catheter dislodgement, pneumothorax, catheter malfunction, hemothorax, or other. Catheter malfunction referred to issues connecting the IPC with the associated drainage system and issues with drainage due to catheter occlusion. Empyema was defined as an infection of the pleural space confirmed by positive pleural fluid cultures or an exudative effusion with clinical signs of infection. Pleural fluid analysis in patients with infectious complications was performed when possible. Pleurodesis following IPC placement was included, defined as resolution of the effusion after IPC placement allowing for catheter removal based on clinician discretion with no additional intervention. Presence of unexpandable lung was recorded and defined as inability of the lung to completely expand after IPC placement despite drainage.20
Data regarding transplant status post-IPC, receipt of liver transplant, IPC removal, hospitalizations within 6 months’ post-IPC placement at our institution, follow-up serum albumin and BMI findings, and death were also collected. Follow-up albumin and BMI data were first collected during the initial hospitalization post-IPC placement to minimize confounding from albumin infusions and additional interventions during the hospital stay. In those patients who were not hospitalized within 6 months, the data points closest to IPC removal were recorded. Death was recorded as a composite outcome that included actual date of death or discharge to hospice care. No additional follow-up data were recorded after discharge to hospice in applicable patients.
Statistical Analysis
Data were collected from medical records and managed by using REDCap electronic data capture tools hosted at the Indiana Clinical and Translational Sciences Institute.21 Descriptive statistics were used to analyze demographic data. Model for End-Stage Liver Disease–Sodium (MELD-Na) scores were calculated from baseline laboratory values.22 Mean ± SD values were reported for all continuous data. Paired Student t tests were used to compare baseline and follow-up albumin and BMI values. The data were analyzed by using SAS version 9.4 (SAS Institute, Inc). Statistical analysis support was provided by the Indiana University Department of Biostatistics.
Results
A total of 62 patients were included in the analysis. The mean age at time of IPC placement was 61 years (range, 35-89 years) (Table 1), and 34 (55%) were male. The most common etiology of cirrhosis was nonalcoholic steatohepatitis (42%), followed by chronic alcohol use (32%). The mean MELD-Na score at the time of IPC placement was 24 (range, 11-38). The majority of effusions were right-sided, and the most common type of catheter placed was the Aspira drain (used in 36 [58%] patients). The majority of patients had received previous therapy with salt restriction, diuretics, and serial thoracentesis. Five patients underwent TIPS prior to IPC placement. Twenty-one patients (34%) received peri-procedural antibiotics.
Table 1.
Demographic Data (N = 62)
| Characteristic | Value |
|---|---|
| Age, y | |
| Mean ± SD | 60.7 ± 10.8 |
| Range | 35-89 |
| Male sex | 34 (54.8%) |
| Affected side, right | 56 (90.3%) |
| MELD-Na score, mean ± SD | 24 ± 6.5 |
| Etiology of cirrhosis | |
| Alcohol | 20 (32.3%) |
| Hepatitis C | 18 (29.0%) |
| NASH | 26 (41.9%) |
| Autoimmune | 2 (3.2%) |
| Alpha1-antitrypsin deficiency | 2 (3.2%) |
| Other | 10 (16.1%) |
| Transplant status, listeda | 7 (11.3%) |
| Indication for IPC | |
| Bridge to transplant | 33 (53.2%) |
| Palliative | 24 (38.7%) |
| Unclear | 5 (8.1%) |
IPC = indwelling pleural catheter; MELD-Na = Model for End-Stage Liver Disease–Sodium; NASH = nonalcoholic steatohepatitis.
At time of IPC placement.
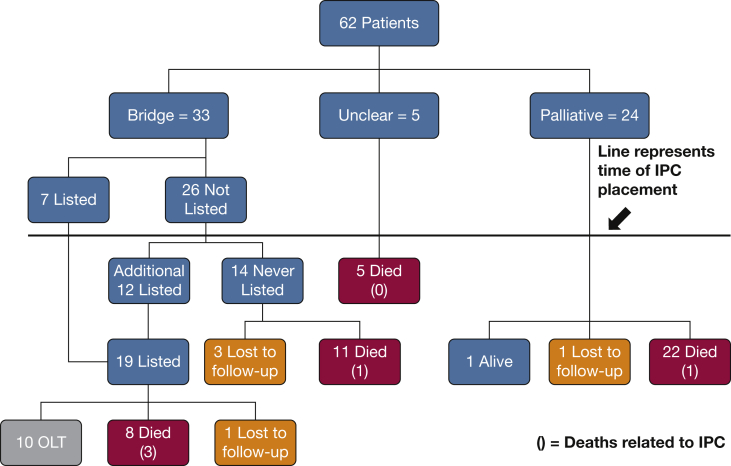
Thirty-three of the 62 patients (53%) had IPCs placed as a bridge to transplant, and 24 patients (39%) had the catheters placed with palliative intent. In the remaining five patients, the context of IPC placement could not be discerned. Twenty-two (35%) of the IPCs were ultimately removed: nine due to pleurodesis (five in the transplant group, four in the unclear group), six following transplantation, and seven due to complications (empyema [n = 2], dislodgement [n = 1], and others [n = 4]). The mean time to pleurodesis was 118 days (range, 15-373 days). Forty-eight of the patients died during the study period, with a mean time to death following IPC placement of 180 days (range, 0-1,258 days). These data included 19 (58%) patients in whom IPC was used as a bridge to transplant. Five patients (8%) were lost to follow-up.
Complications
Catheter-related complications occurred in 22 patients (Table 2). The most serious complication was empyema, recorded in 10 patients (16.1%). In three of these patients, death was directly related to the empyema. Specific details regarding patients with empyema are presented in Table 3. Other notable complications include catheter dislodgement (n = 6), pneumothorax (n = 2), and cellulitis (n = 1). Unexpandable lung was diagnosed in three patients following IPC placement, two of whom were successfully listed and received a liver transplant, with IPC removal at the time of transplant or shortly thereafter. The third patient was listed following IPC placement but experienced additional complications, including infection of the pleural space, and died in the ICU of multiorgan dysfunction syndrome prior to undergoing transplantation.
Table 2.
Outcomes of IPC Placement
| Outcome | Value |
|---|---|
| Complications | 22 (35.5%) |
| Type of complication | |
| Empyema | 10 (16.1%) |
| Skin infection | 1 (1.6%) |
| Catheter clogged | 2 (3.2%) |
| Catheter dislodged | 6 (9.7%) |
| Pneumothorax | 2 (3.2%) |
| Catheter malfunction | 5 (8.1%) |
| Other | 3 (4.8%) |
| Unexpandable lung | 3 (4.8%) |
| Pleurodesis | 9 (14.5%) |
| Time to pleurodesis, d | |
| Mean ± SD | 118 ± 139.6 |
| Range | 15-373 |
| Transplant status after IPCa,b | |
| Listed | 19 (57.6%) |
| Not listed | 14 (42.4%) |
| Transplant after IPCb | 10 (30.3%) |
| Time to transplant, d | |
| Mean ± SD | 87 ± 49.6 |
| Range | 20-175 |
| Death after placement | 48 (77.4%) |
| Time to death, d | |
| Mean ± SD | 180 ± 284.0 |
| Range | 0-1,258 |
| Death at 6 months | 36 (58%) |
| Lost to follow-up | 5 (8.1%) |
See Table 1 legend for expansion of abbreviation.
Excludes palliative patients.
Percentages calculated as fraction of transplant-eligible patients.
Table 3.
Patients With Pleural Infection
| Indication | PA | Removed | Transplant | Deatha | LDH Fluidb (U/L) | Organism |
|---|---|---|---|---|---|---|
| Bridge | Yes | No | No | Yes | 37 | Coagulase-negative Staphylococcus |
| Palliative | No | No | … | No | 162b | Acinetobacter baumannii, methicillin-resistant Staphylococcus aureus |
| Bridge | No | Yes | Yes | No | 152b | No organism identified |
| Bridge | No | Yes | No | No | 206b | Coagulase-negative Staphylococcus |
| Palliative | No | No | … | Yes | N/A | Escherichia coli |
| Unclear | No | No | … | No | 106 | Klebsiella pneumoniae, Corynebacterium species |
| Bridge | No | Yes | Yes | No | 80b | Corynebacterium species, Streptococcus agalactiae |
| Bridge | No | No | No | Yes | 54 | K pneumoniae |
| Bridge | No | No | No | No | 133b | Coagulase-negative Staphylococcus, Corynebacterium species, A baumannii, Enterococcus species |
| Bridge | No | No | No | No | 57 | Klebsiella oxytoca |
LDH = lactate hydrogenase; PA = peri-procedural antibiotic.
Death due to infection.
Exudate per Light’s criteria.
Transplant Outcomes
Thirty-three patients were under consideration for liver transplantation at the time of IPC placement (Fig 1). Of those, seven patients were actively listed for transplantation when the catheter was placed. Twelve additional patients were listed following IPC placement, for a total of 19 patients listed for liver transplantation. Of these, 10 underwent successful transplantation, with a mean time to transplant following IPC placement of 87 days (range, 20-175 days). In six patients, the IPC was removed following transplantation, and in three it was removed prior to transplantation. One of these three patients developed an empyema, recovered, and underwent successful transplantation. One patient died of refractory shock, with the IPC still in place in the days following transplantation. Of the remaining nine patients who were listed, one was lost to follow-up, and eight died while awaiting transplantation. Of these eight patients, two died of septic shock related to empyema; the others died of various complications of end-stage liver disease that seemed unrelated to the IPC. Of the original 33 patients under consideration for transplantation at the time of IPC placement, 14 were never listed for transplantation.
Figure 1.
Patient flowchart. The number of deaths related to IPC is given in parentheses. IPC = indwelling pleural catheter; OLT = orthotopic liver transplantation.
Effect on Albumin and BMI
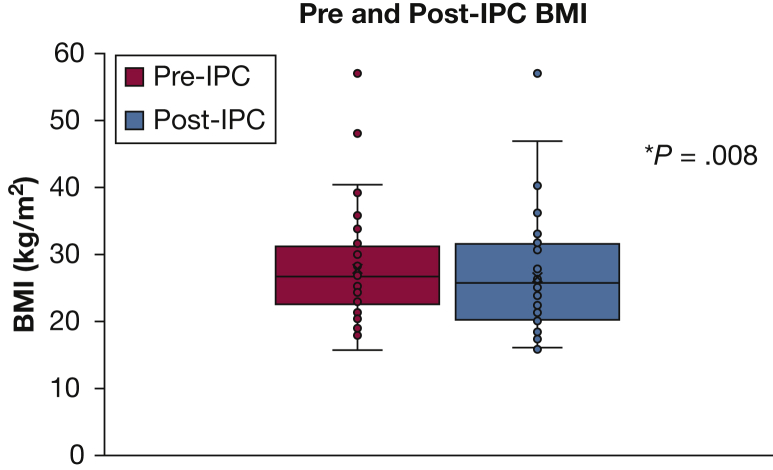
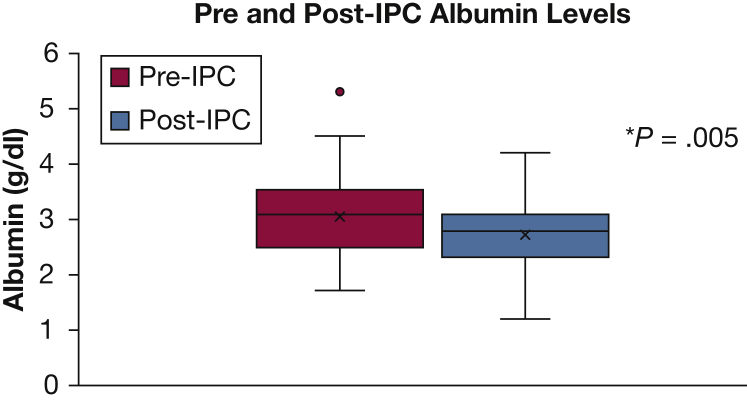
The mean baseline BMI was 27.8 kg/m2 (n = 56; range, 15.7-57.1 kg/m2) (Fig 2). The mean follow-up BMI was 26.7 kg/m2 (n = 53; range, 16.0-57.1 kg/m2), with a mean time to follow-up of 32.6 days (range, 1-141 days). The mean difference between pre-IPC and post-IPC BMI values was a decrease of 1.13 kg/m2 (n = 50), which reached statistical significance (P = .008). The mean baseline serum albumin level was 3.0 g/dL (n = 59; range, 1.7-5.3 g/dL), with mean follow-up level of 2.7 g/dL (n = 55; range, 1.2-4.2 g/dL) (Fig 3). The mean time to follow-up for albumin was 29.6 days (range, 1-122 days). The mean difference between pre-IPC and post-IPC values was a decrease of 0.3 g/dL (n = 53), which also reached statistical significance (P = .005).
Figure 2.
BMI prior to and following IPC. *Statistically significant. See Figure 1 legend for expansion of abbreviation.
Figure 3.
Albumin levels prior to and following IPC. *Statistically significant. See Figure 1 legend for expansion of abbreviation.
Discussion
IPCs are now accepted for the management of symptomatic malignant pleural effusions,12, 13 and their use has recently been expanded to many forms of benign pleural effusion.14, 15 We report the largest study to date assessing outcomes of IPCs in patients with medically refractory HH at a large tertiary liver transplantation center. The majority of the patients included in this study had either failed to improve with previous TIPS or were not considered candidates for TIPS, necessitating alternate means of controlling their HH. Common contraindications to TIPS include history of hepatic encephalopathy, hyperbilirubinemia, pulmonary hypertension, severe congestive heart failure, and structural lesions of the liver that may preclude placement of the shunt.22
Our study revealed complications in 36% of patients. A recent review of 325 patients with benign pleural effusion who underwent IPC placement reported a complication rate of 17% in the study population.15 Notably, this review included a majority of patients with cardiac and renal-related pleural effusions, with only a minority of cases (12%) with liver disease. Our complication rate was considerably higher. One possible explanation for this finding is the natural course of patients with end-stage liver disease, including high rates of infection and death.5 This theory is supported by our finding that the mean MELD-Na score at the time of IPC placement in this study population was 24, which suggests a 3-month mortality rate of approximately 20%.23
The most important complication of IPCs was empyema, diagnosed in 10 patients (16.1%). This rate is higher than that reported thus far in patients with IPCs placed following solid organ transplantation (11%) and in those with hematologic malignancies undergoing chemotherapy (5.2%-7.7%).24, 25, 26 However, this rate is almost identical to that seen in a prospective feasibility study (16.7%) assessing IPCs as a bridge-to-transplant strategy in patients with HH at a separate liver transplant referral institution.18 This number is also comparable to a retrospective analysis of 508 patients with cirrhosis and HH treated with thoracentesis in which the incidence of spontaneous bacterial empyema was 15.9%.27 In our study, three cases (4.8%) of empyema necessitated IPC removal, and in three cases (4.8%), the empyema precipitated septic shock and death; thus, it represents a serious concern in patients being considered for IPC placement.
It should be noted that many of the pleural infections identified in this study likely represent spontaneous bacterial empyema, whereby bacterial infection of the pleural space occurs via translocation of enteric bacteria into the pleural space.28 Thus, the infections may not have been directly caused by the catheters themselves. This theory is supported by the predominance of gram-negative and enteric pathogens isolated from pleural cultures. Patients with HH have demonstrably lower complement levels and decreased opsonic activity in pleural fluid compared with patients with effusions of other causes, which may predispose this population to pleural space infections.29 Previous data in patients with IPCs for malignant pleural effusions showed that Staphylococcus aureus was the most common bacteria isolated from pleural cultures, and patients with gram-negative pathogens had worse outcomes, including death; in this cohort, the majority of patients were successfully treated without IPC removal.30 In addition, four of the 10 patients with pleural space infections in our study had transudative effusions. Previous data have also shown that traditional markers such as fluid lactate dehydrogenase levels may be a less reliable marker of infection in patients with HH who develop spontaneous bacterial empyema.31 Thus, the significance of transudative effusions with positive cultures in the setting of an IPC is less clear. Lastly, some of the pleural fluid isolates in our study represented common skin flora, and we cannot rule out that in these cases, there was simply contamination rather than true infection. This theory would lower our true infection rate to more closely align with other recent studies. However, because these patients were treated with antibiotics, infection was assumed.
Ten of the 33 patients (30%) in the present study who were considered eligible for transplantation went on to successfully receive a liver transplant, which is similar to the 25% of patients who ultimately underwent transplantation in a smaller pilot study of IPC feasibility for medically refractory HH.18 In our study, IPCs were successfully removed in all transplanted patients with the exception of one patient who developed significant postoperative complications unrelated to the IPC and ultimately died. We did identify two patients who developed empyema while they were actively listed, and both died of septic shock, demonstrating the risk that IPC-related complications may preclude liver transplantation.
Previous studies have cited protein loss and electrolyte abnormalities as complications of traditional tube thoracostomy in HH.10, 11 We observed statistically significant decreases in both measures, although the absolute decreases were small and of questionable clinical significance. Furthermore, the lack of a control group prevented us from assessing whether these effects were truly related to the IPC or were simply manifestations of progressive end-stage liver disease.
Our study has several limitations. First, the retrospective and observational nature of this study means the data are subject to bias and limits the strength of our conclusions. All records were reviewed in detail; however, only follow-up data that were available in our institution’s records could be obtained, and thus it is possible that additional complications could have occurred outside of our network. A small portion (8%) of the patients were ultimately lost to follow-up, including one patient who was actively listed for liver transplantation; thus, their final outcomes are unknown. The albumin and BMI data may also be subject to bias, and it is possible that data might have been influenced by factors that were not specifically captured in the electronic medical record. Our results may be subject to selection bias, and it is possible we did not identify all patients who could have benefited from an IPC. Lastly, patients in this study were not given a standardized drainage protocol, which has shown benefit in patients with malignant pleural effusion.32
Conclusions
We present our single-center experience with IPC use in patients with medically refractory HH. We believe that IPCs can serve as an effective means of palliation in patients with end-stage liver disease who are not transplant candidates and are experiencing refractory hydrothorax. All such patients should be counseled on the potential risks of this approach, including infection. We believe IPCs should be used with caution in patients who are eligible for liver transplantation. Although we reported success with IPCs as a bridge to transplant, we also observed cases in which complications of the IPC actually prevented patients being able to undergo transplantation. Patients who are eligible for transplant and experiencing HH who have failed to improve with traditional treatment strategies would likely benefit from a multidisciplinary approach to care, including input from experts in the fields of hepatology, pulmonology, and transplant surgery. Prospective studies would help to identify the ideal patient population. Future controlled studies are also needed to better assess the effects of this approach with regard to infection risk, nutritional status, and use in transplant patients.
Acknowledgments
Author contributions: C. K. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. C. K. contributed to the conception and design of the study protocol; collection, analysis, and interpretation of the data; drafting and revision of the manuscript; and generation of the tables and figures. K. D. contributed to the collection, analysis, and interpretation of the data; and drafting and revision of the manuscript. M. G. contributed to the conception and design of the study protocol; analysis and interpretation of the data; and drafting and revision of the manuscript. G. B. contributed to the conception and design of the study protocol; collection, analysis, and interpretation of the data; drafting and revision of the manuscript; and generation of the tables and figures. All authors approved the final manuscript.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This project was supported, in part, by the Indiana Clinical and Translational Sciences Institute, funded in part by National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award from the National Institutes of Health [award number UL1TR001108]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Alonso J.C. Pleural effusion in liver disease. Semin Respir Crit Care Med. 2010;31(6):698–705. doi: 10.1055/s-0030-1269829. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Tsao G., Lim J.K. Management and treatment of patients with cirrhosis and portal hypertension: recommendations from the Department of Veterans Affairs Hepatitis C Resource Center Program and the National Hepatitis C Program. Am J Gastroenterol. 2009;104(7):1802–1829. doi: 10.1038/ajg.2009.191. [DOI] [PubMed] [Google Scholar]
- 3.Machicao V.I., Balakrishnan M., Fallon M.B. Pulmonary complications in chronic liver disease. Hepatology. 2014;59(4):1627–1637. doi: 10.1002/hep.26745. [DOI] [PubMed] [Google Scholar]
- 4.Badillo R., Rockey D.C. Hepatic hydrothorax: clinical features, management, and outcomes in 77 patients and review of the literature. Medicine (Baltimore) 2014;93(3):135–142. doi: 10.1097/MD.0000000000000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu W.L., Kuo P.H., Ku C.H., Huang P.M., Yang P.C. Impact of therapeutic interventions of patients with hepatic hydrothorax. J Formos Med Assoc. 2010;109(8):582–588. doi: 10.1016/S0929-6646(10)60095-2. [DOI] [PubMed] [Google Scholar]
- 6.Porcel J.M. Management of refractory hepatic hydrothorax. Curr Opin Pulm Med. 2014;20(4):352–357. doi: 10.1097/MCP.0000000000000058. [DOI] [PubMed] [Google Scholar]
- 7.Conklin L.D., Estrera A.L., Weiner M.A., Reardon P.R., Reardon M.J. Transjugular intrahepatic portosystemic shunt for recurrent hepatic hydrothorax. Ann Thorac Surg. 2000;69(2):609–611. doi: 10.1016/s0003-4975(99)01351-x. [DOI] [PubMed] [Google Scholar]
- 8.Xiol X., Tremosa G., Castellote J. Liver transplantation in patients with hepatic hydrothorax. Transpl Int. 2005;18(6):672–675. doi: 10.1111/j.1432-2277.2005.00116.x. [DOI] [PubMed] [Google Scholar]
- 9.Runyon B.A., Greenblatt M., Ming R.H. Hepatic hydrothorax is a relative contraindication to chest tube insertion. Am J Gastroenterol. 1986;81(7):566–567. [PubMed] [Google Scholar]
- 10.Liu L.U., Haddadin H.A., Bodian C.A. Outcome analysis of cirrhotic patients undergoing chest tube placement. Chest. 2004;126(1):142–148. doi: 10.1378/chest.126.1.142. [DOI] [PubMed] [Google Scholar]
- 11.Orman E.S., Lok A.S. Outcomes of patients with chest tube insertion for hepatic hydrothorax. Hepatol Int. 2009;3(4):582–586. doi: 10.1007/s12072-009-9136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tremblay A., Michaud G. Single-center experience with 250 tunnelled pleural catheter insertions for malignant pleural effusion. Chest. 2006;129(2):362–368. doi: 10.1378/chest.129.2.362. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki K., Servais E.L., Rizk N.P. Palliation and pleurodesis in malignant pleural effusion: the role for tunneled pleural catheters. J Thorac Oncol. 2011;6(4):762–767. doi: 10.1097/JTO.0b013e31820d614f. [DOI] [PubMed] [Google Scholar]
- 14.Bhatnagar R., Reid E.D., Corcoran J.P. Indwelling pleural catheters for non-malignant effusions: a multicentre review of practice. Thorax. 2014;69(10):959–961. doi: 10.1136/thoraxjnl-2013-204563. [DOI] [PubMed] [Google Scholar]
- 15.Patil M., Dhillon S.S., Attwood K., Saoud M., Alraiyes A.H., Harris K. Management of benign pleural effusions using indwelling pleural catheters: a systematic review and meta-analysis. Chest. 2017;151(3):626–635. doi: 10.1016/j.chest.2016.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Mercky P., Sakr L., Heyries L., Lagrange X., Sahel J., Dutau H. Use of a tunnelled pleural catheter for the management of refractory hepatic hydrothorax: a new therapeutic option. Respiration. 2010;80(4):348–352. doi: 10.1159/000282493. [DOI] [PubMed] [Google Scholar]
- 17.Shah R., Succony L., Gareeboo S. Use of tunneled pleural catheters for the management of refractory hepatic hydrothorax. BMJ Case Rep. 2011;2011 doi: 10.1136/bcr.05.2011.4213. pii: bcr0520114213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen A., Massoni J., Jung D., Crippin J. indwelling tunneled pleural catheters for the management of hepatic hydrothorax: a pilot study. Ann Am Thorac Soc. 2016;13(6):862–866. doi: 10.1513/AnnalsATS.201510-688BC. [DOI] [PubMed] [Google Scholar]
- 19.Spector M., Pollak J.S. Management of malignant pleural effusions. Semin Respir Crit Care Med. 2008;29(4):405–413. doi: 10.1055/s-2008-1081283. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y.S., Susanto I., Lazar C.A. Pneumothorax ex-vacuo or “trapped lung” in the setting of hepatic hydrothorax. BMC Pulm Med. 2012;12:78. doi: 10.1186/1471-2466-12-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyer T.D., Haskal Z.J. The role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension. Hepatology. 2005;41(2):386–400. doi: 10.1002/hep.20559. [DOI] [PubMed] [Google Scholar]
- 23.Kim W.R., Biggins S.W., Kremers W.K. Hyponatremia and mortality among patients on the liver-transplant waiting list. N Engl J Med. 2008;359(10):1018–1026. doi: 10.1056/NEJMoa0801209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skalski J.H., Pannu J., Sasieta H.C., Edell E.S., Maldonado F. Tunneled indwelling pleural catheters for refractory pleural effusions after solid organ transplant. Ann Am Thorac Soc. 2016;13(8):1294–1298. doi: 10.1513/AnnalsATS.201601-080BC. [DOI] [PubMed] [Google Scholar]
- 25.Mekhaiel E., Kashyap R., Mullon J.J., Maldonado F. Infections associated with tunneled indwelling pleural catheters in patients undergoing chemotherapy. J Bronchol Intervent Pulmonol. 2013;20(4):299–303. doi: 10.1097/LBR.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert C.R., Lee H.J., Skalski J.H. The use of indwelling tunneled pleural catheters for recurrent pleural effusions in patients with hematologic malignancies: a multicenter study. Chest. 2015;148(3):752–758. doi: 10.1378/chest.14-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen C.H., Shih C.M., Chou J.W. Outcome predictors of cirrhotic patients with spontaneous bacterial empyema. Liver Int. 2011;31(3):414–424. doi: 10.1111/j.1478-3231.2010.02447.x. [DOI] [PubMed] [Google Scholar]
- 28.Xiol X., Castellvi J.P., Guardiola J. Spontaneous bacterial empyema in cirrhotic patients: a prospective study. Hepatology. 1996;23(4):719–723. doi: 10.1002/hep.510230410. [DOI] [PubMed] [Google Scholar]
- 29.Sese E., Xiol X., Castellote J., Rodriguez-Farinas E., Tremosa G. Low complement levels and opsonic activity in hepatic hydrothorax: its relationship with spontaneous bacterial empyema. J Clin Gastroenterol. 2003;36(1):75–77. doi: 10.1097/00004836-200301000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Fysh E.T., Tremblay A., Feller-Kopman D. Clinical outcomes of indwelling pleural catheter-related pleural effusions: an international multicenter study. Chest. 2013;144(5):1597–1602. doi: 10.1378/chest.12-3103. [DOI] [PubMed] [Google Scholar]
- 31.Gurung P., Goldblatt M., Huggins J.T., Doelken P., Nietert P.J., Sahn S.A. Pleural fluid analysis and radiographic, sonographic, and echocardiographic characteristics of hepatic hydrothorax. Chest. 2011;140(2):448–453. doi: 10.1378/chest.10-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wahidi M.M., Reddy C., Yarmus L. Randomized trial of pleural fluid drainage frequency in patients with malignant pleural effusions—the ASAP Trial. Am J Resp Crit Care Med. 2017;195(8):1050–1057. doi: 10.1164/rccm.201607-1404OC. [DOI] [PubMed] [Google Scholar]