Abstract
Background
Skeletal muscle dysfunction occurring as a result of ICU admission associates with higher mortality. Although preadmission higher BMI correlates with better outcomes, the impact of baseline muscle and fat mass has not been defined. We therefore investigated the association of skeletal muscle and fat mass at ICU admission with survival and disability at hospital discharge.
Methods
This single-center, prospective, observational cohort study included medical ICU (MICU) patients from an academic institution in the Unites States. A total of 401 patients were evaluated with pectoralis muscle area (PMA) and subcutaneous adipose tissue (SAT) determinations conducted by CT scanning at the time of ICU admission, which were later correlated with clinical outcomes accounting for potential confounders.
Results
Larger admission PMA was associated with better outcomes, including higher 6-month survival (OR, 1.03; 95% CI, 1.01-1.04; P < .001), lower hospital mortality (OR, 0.96; 95% CI, 0.93-0.98; P < .001), and more ICU-free days (slope, 0.044 ± 0.019; P = .021). SAT was not significantly associated with any of the measured outcomes. In multivariable analyses, PMA association persisted with 6 months and hospital survival and ICU-free days, whereas SAT remained unassociated with survival or other outcomes. PMA was not associated with regaining of independence at the time of hospital discharge (OR, 0.99; 95% CI, 0.98-1.01; P = .56).
Conclusions
In this study cohort, ICU admission PMA was associated with survival during and following critical illness; it was unable to predict regaining an independent lifestyle following discharge. ICU admission SAT mass was not associated with survival or other measured outcomes.
Key Words: adipose tissue, critical illness, frailty, muscle wasting, pectoralis muscle area
Abbreviations: MICU, medical ICU; mMRC, modified Medical Research Council; PMA, pectoralis muscle area; SAT, subcutaneous adipose tissue
Skeletal muscle dysfunction, which encompasses wasting and weakness,1, 2 is associated with ICU mortality,3, 4 failure to wean from mechanical ventilation,5 and ICU readmission.6 In ICU survivors, muscle dysfunction persists for years despite the full recovery of other organ functions,7, 8 and postdischarge weight gain occurs primarily by an increase in fat and not lean body mass.9 Baseline skeletal muscle mass reflects nutritional status, which is associated with survival as well.10
Although the relevance of muscle wasting occurring during1, 5, 11, 12 and following7, 8, 13 ICU admission is well established, there are no substantial data regarding the association of preadmission muscle mass with survival and other clinically relevant outcomes. Previous retrospective data suggest that larger peri-admission muscle mass and protein content are associated with improved prognosis14, 15; however, muscle mass was determined in those reports up to 4 days following ICU admission when significant muscle wasting may have already occurred.1 Thus, these studies did not specifically address the association of pre-ICU admission muscle mass with important clinical outcomes. Moreover, although evidence indicates that preadmission body weight influences ICU outcomes,16 the independent contribution of baseline muscle and fat mass to the survival of ICU patients remains unclear.
The present study analyzed the association of muscle and fat mass determined within 24 h of ICU admission with survival and other outcomes. Our central hypothesis was that patients with greater admission muscle mass would have a better prognosis compared with wasted individuals and that adipose tissue mass would be relatively less relevant on patients’ outcomes. To test that hypothesis, data were collected on the muscle mass using the surrogate of the pectoralis muscle area (PMA) as determined according to chest CT scans performed within the first 24 h of ICU admission. Fat mass was assessed on these chest CT scans by using the surrogate of subcutaneous adipose tissue (SAT) area at the level of seventh to the eighth thoracic vertebral body (T7-T8). We also collected follow-up data on the patients’ clinical outcomes over time. Some of these results have been previously reported in abstract form.17
Patients and Methods
This prospective, single-center, observational study included adult subjects admitted to the medical ICU (MICU) of Albany Medical Center. Ethical approval was obtained from the Albany Medical College Committee on Research Involving Human Subjects (institutional review board no. 4281). Enrollment occurred between November 2015 and February 2017. Patients were considered for enrollment if they were aged > 18 years, admitted to the MICU, required a chest CT scan within the first 24 h of MICU admission, and were anticipated to require an ICU stay > 24 h. Exclusion criteria were primary neuromuscular pathology, acute illness leading to imminent death, or chronic illness with a life expectancy < 6 months. Enrollment was attempted on all eligible patients, and written consent was obtained from the patient or a legally authorized representative.
Preliminary analysis of the data indicated that about 112 events would need to occur to reach a significant effect of PMA on mortality at 6 months, which led to a cohort size of about 400 patients given a mortality rate of 30% and a 90% follow-up efficiency at the time of that analysis. Enrolled patients were monitored daily for survival, length of ICU stay, and disposition at discharge. Survival at 6 months was determined either by hospital documentation or telephone communication.
PMA measurements were performed as previously established18 by using a single axial slice of the CT scan with in-house software. In short, a single reader blinded to patients’ identity and clinical characteristics visually identified the first axial image above the superior aspect of the aortic arch. Muscles were manually shaded by using a predefined attenuation range of –50 and 90 HU, and the PMA was computed as the aggregated area in square centimeters of the right and left pectoralis major and minor muscles assessed in this axial plane (Fig 1). The intraobserver PMA measurement variability was determined in 20 CT scans randomly selected that were remeasured at least 6 months apart; differences were found to be not significantly different from zero (bias = – 0.0875 cm2 with limits of agreement ±0.54 cm2) (e-Table 1). SAT was measured as previously described, at the level of T7-T8 thoracic vertebral body19 (e-Fig 1). A more detailed explanation of methods can be found in e-Appendix 1.
Figure 1.
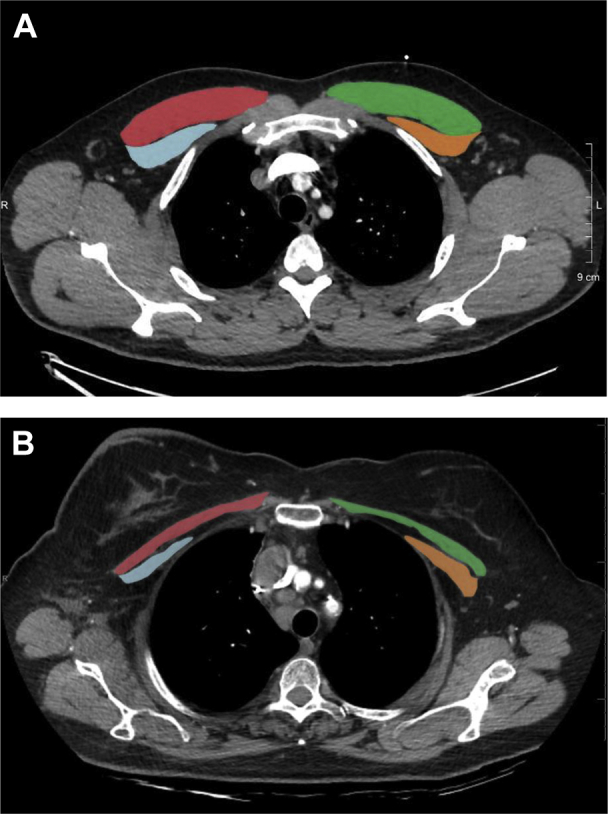
Sample CT scans used to determine muscle area in our cohort. A, Relatively nonwasted. B, Relatively wasted. Red indicates right pectoralis major muscle; green indicates left pectoralis major muscle; blue indicates right pectoralis minor muscle; and yellow indicates left pectoralis minor muscle.
Statistical Analysis
The primary outcome variable was 6-month survival, and secondary outcomes were hospital survival, disability at hospital discharge, and ICU-free days to day 28, which is a composite outcome that aggregates length of ICU stay and survival (patients who stay in the ICU for ≥ 28 days and patients who die in the ICU are given a score of 0).20 We chose different covariates a priori based on previous literature and clinical relevance, and then assessed how they associated with categorical outcome measures by using logistic regression models. To study how continuous outcomes measures are explained by covariates, linear regression models were fit. Univariate and multivariable logistic regressions were performed by using Minitab Statistical Software (Minitab, Inc.) with significance accepted at a two-tailed alpha of 0.05.
To build the multivariable models, we determined how covariates were related to the outcome of interest via correlations in the first stage. Those that were associated with a significant P value ≤ .2 were retained, and standard stepwise model selection routines were then followed. To assess goodness of fit of the multivariable models, Akaike information criteria as well as likelihood ratio test statistics were compared. We also checked the plausibility of linearity as well as normality assumptions. Both Acute Physiology and Chronic Health Evaluation II and Sequential Organ Failure Assessment scores were calculated in every patient, but given the strong collinearity among them and to minimize overfitting, only the Sequential Organ Failure Assessment scores were included in the multivariable analysis.
Disability at discharge was analyzed as discharge location with three possible outcomes: (1) in-hospital death; (2) discharge to a facility or home with assisted living required (discharged not independent); or (3) discharge to home without assisted living required (discharged independent). These variables were analyzed by using ORs for these outcomes, which were determined by nominal logistic regression. Comparison of PMA and SAT among these three groups was performed according to ANOVA with Tukey test for multiplicity. Several post hoc analyses were performed to further investigate interaction effects related to sex.
Results
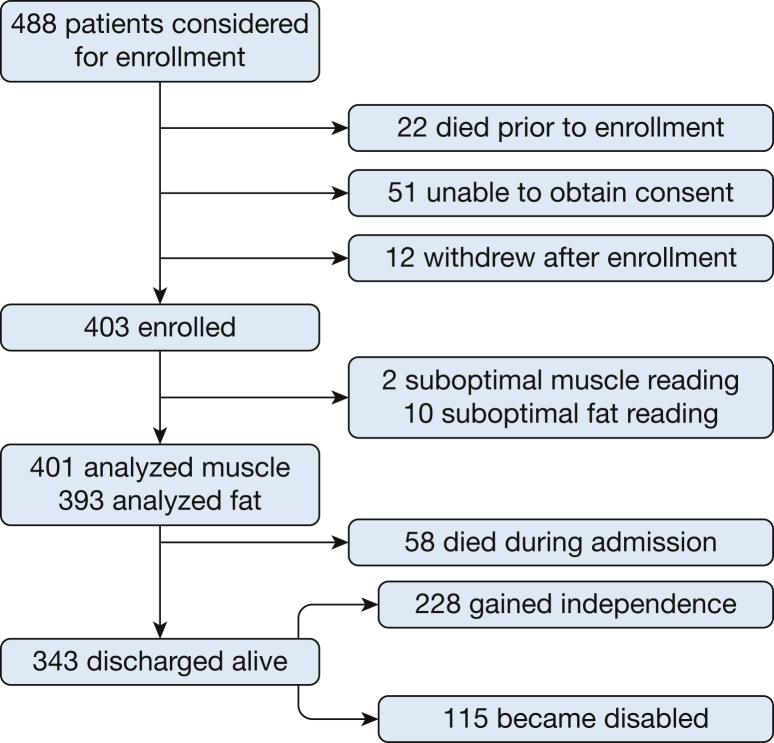
A total of 488 MICU patients were considered eligible for this study, of whom 403 (83%) were included, and 85 (17%) were excluded (Fig 2). Two patients were later excluded due to technical limitations in determining muscle area on CT imaging, leaving 401 patients who were analyzed. SAT was technically not measurable in 10 CT scans, which left 393 patients analyzed for that variable. The demographic data are shown in Table 1. Median PMA was 43.9 cm2 with an interquartile range of 34.7 to 56.2 cm2 and an overall range of 12.7 to 181.4 cm2. Median SAT was 20.1 cm2 (interquartile range of 11.7-26.8 cm2 and an overall range of 1.45 to 57.6 cm2). The average age of the cohort was 62 years, and there was a slight male predominance (55.6%), and consistent with previous reports,18 PMA was weakly inversely correlated with age (e-Fig 2). Of the 401 patients enrolled and analyzed, 58 (14%) died in the hospital, and 343 (85%) were discharged alive. Among these survivors, 228 (66%) regained independence, and 115 (33%) were not independent at discharge. At 6 months, 266 patients were alive, 127 had died, and eight could not be reached to determine status. Data on these eight missing patients were not included in the 6-month survival analyses. Most of the patients (61%) underwent CT chest scanning to investigate possible pulmonary embolism (32%), respiratory distress (15%), or suspected pneumonia/atelectasis (14%) (e-Table 2). Information regarding the primary cause of ICU admission of patients from the study cohort compared with the general ICU population during the enrollment period is presented in e-Tables 3 through 5.
Figure 2.
Enrollment flowchart.
Table 1.
Patients’ Baseline Characteristics
| Characteristic | Value |
|---|---|
| Male sex | 55.6% |
| PMA, cm2a | 43.9 (34.7-56.2) |
| SAT, cm2 | 20.1 (11.7-26.8) |
| Age, y | 62 (50.7-72.2) |
| Severity scores | |
| APACHE II | 16 (11-22) |
| SOFA | 5.23 (3-7) |
| Preadmission comorbidities | |
| Pulmonary disease | 157 (38%) |
| Steroid use | 104 (25%) |
| Diabetes | 101 (25%) |
| Cancer | 90 (22%) |
| Congestive heart failure | 77 (19%) |
| End-stage renal disease | 33 (8.1%) |
| Preadmission mMRC score | 2 (1-4) |
| Primary reason for ICU admission | |
| Respiratory failure | 171 (42%) |
| Nonrespiratory sepsis | 71 (17%) |
| Pulmonary embolism | 66 (16%) |
| Hemorrhagic shock | 22 (5.4%) |
| Altered mental status | 21 (5.1%) |
| Metabolic cause, including diabetic ketoacidosisb | 13 (3.2%) |
| Trauma | 11 (2.7%) |
| Cardiovascular decompensation | 15 (3.7%) |
| Other | 6 (1.4%) |
Data are presented as median (interquartile range) unless otherwise indicated. APACHE II = Acute Physiology and Chronic Health Evaluation II; mMRC = Modified Medical Research Council; PMA = pectoralis muscle area; SAT = subcutaneous adipose tissue; SOFA = Sequential Organ Failure Assessment.
PMA values for female subjects were multiplied by 1.67 in this analysis.
Includes toxicologic problems.
Effect of Admission PMA on Outcomes
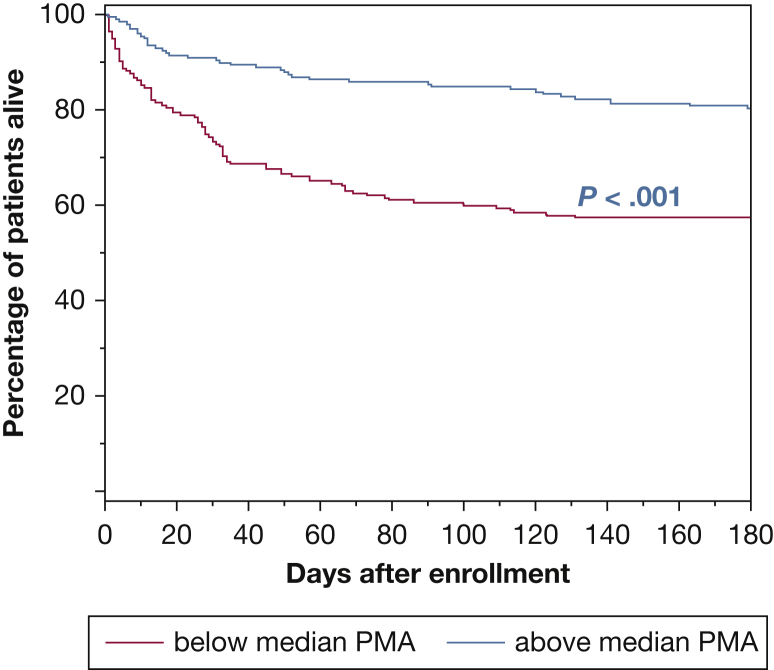
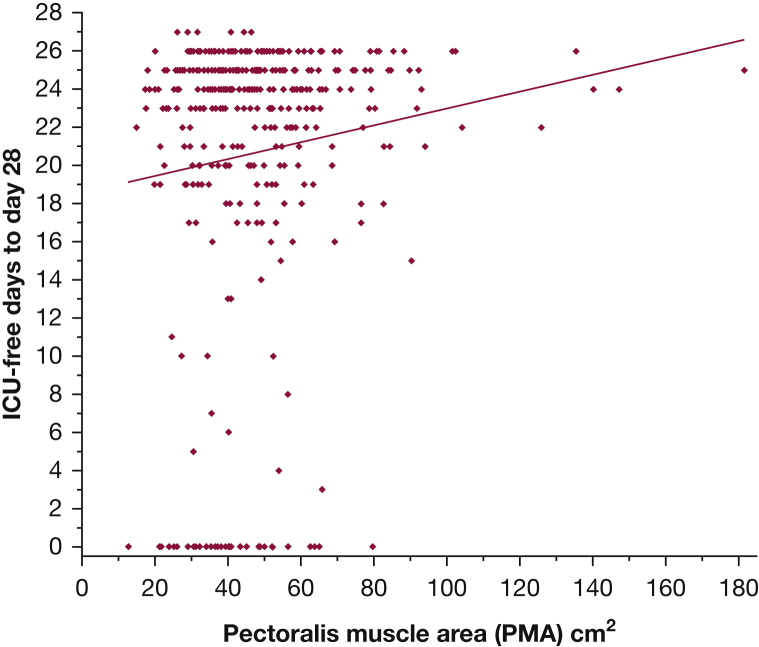
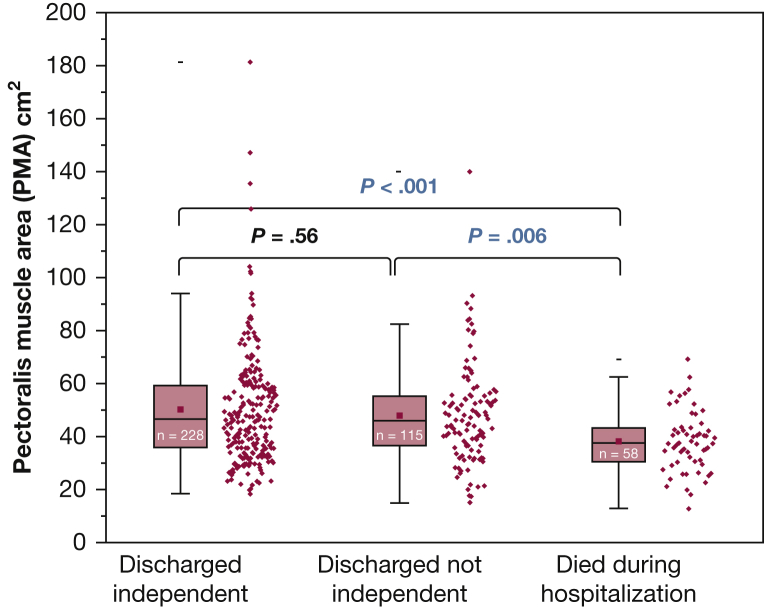
Larger PMA was significantly associated with the odds of survival at 6 months (OR, 1.03 per cm2 increase in PMA; 95% CI, 1.01-1.04; P < .001). Figure 3 illustrates the survival difference between patients whose muscle area was above or below the median PMA value (log-rank test, P < .001). Also, larger PMA was significantly associated with lower likelihood of in-hospital death (OR, 0.96 per cm2 PMA; 95% CI, 0.93-0.98; P < .001) (Table 2). Although larger PMA associated with more ICU-free days to day 28 (slope, 0.044 ± 0.019 days per cm2 PMA; P = .021; r2 = 0.013) (Fig 4), it was not associated with independent vs nonindependent living immediately following hospital discharge (OR, 0.99 per cm2 PMA; 95% CI, 0.98-1.01; P = .56) (Fig 5). In a multivariable analysis, the association of PMA with 6-month and hospital survival, and with ICU-free days, persisted even after adjusting for other covariables, including severity score and admission albumin (Table 3, e-Tables 6, 7, 11-15); and it remained not associated with disability at hospital discharge (e-Table 8).
Figure 3.
Kaplan-Meier survival at 6 months: Comparison of survival rate, expressed as percentage of patients alive over time, between patients with PMA below (red) and above (blue) the median value of 43.9 cm2. At 6 months, 266 patients were alive, 127 had died, and eight could not be reached to determine status. P < .001, log-rank test. Pectoralis MA values for female patients were multiplied by 1.67 in this analysis. MA = muscle area.
Table 2.
Univariate Associations Between PMA and SAT With Primary and Secondary Outcomes
| Variable | ICU-Free Days to Day 28 | Disability at Discharge | Hospital Mortality | 6-Month Survival |
|---|---|---|---|---|
| PMAa | Slope, 0.044 ± 0.019; P = .021 | OR, 0.99 (0.98-1.01), P = .56 | OR, 0.96 (0.93-0.98), P < .001 | OR, 1.03 (1.01-1.04), P < .001 |
| SATb | Slope, -0.001 ± 0.003; P = .68 | OR, 1.00 (1.00-1.00), P = .40 | OR, 0.99 (0.998-1.001), P = .32 | OR, 0.99 (0.997-1.001), P = .32 |
Significant P values shown in bold font. See Table 1 legend for expansion of abbreviations.
PMA values for female subjects were multiplied by 1.67 in this analysis.
SAT measured at the T7-T8 level.
Figure 4.
Correlation between PMA and ICU-free days to day 28. Slope, 0.044 ± 0.019 days per cm2 PMA; P = .021; r2 = 0.013. PMA values for female patients were multiplied by 1.67 in this analysis. PMA = pectoralis muscle area.
Figure 5.
Distribution of PMA stratified according to patient disposition at discharge. Comparison among three groups was done by ANOVA with Tukey test for multiplicity. The groups are different (ANOVA, P < .001), with died in hospital vs discharged independent (P < .001), died in hospital vs discharged not independent (P = .006), and discharged independent vs not independent (P = .56). (Definition of discharged independent and not independent are given in the Patients and Methods section.) PMA values for female patients were multiplied by 1.67 in this analysis. For box plots, center line is median, upper and lower lines are 75th and 25th percentiles, and whiskers are the nonoutlier range (< 1.5 interquartile range from box). See Figure 4 legend for expansion of abbreviation.
Table 3.
Multivariable Risk Factors for 6-Month Mortality
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| PMA (muscle area, per cm2) | 0.98 | 0.968-0.999 | .007 |
| SOFA score | 1.17 | 1.089-1.268 | < .001 |
| Albumin | 0.389 | 0.264-0.574 | < .001 |
| Age | 1.0124 | 0.995-1.029 | .153 |
| SAT | 1.0003 | 0.997-1.0029 | .785 |
| mMRC = 1 | Reference | NA | < .001 |
| mMRC = 2 | 1.93 | 0.86-4.35 | |
| mMRC = 3 | 2.06 | 0.96-3.42 | |
| mMRC = 4 | 4.39 | 2.18-8.83 | |
| mMRC = 5 | 4.33 | 1.62-6.87 |
Significant P values shown in bold font. See Table 1 legend for expansion of abbreviations.
aPMA values for female subjects were multiplied by 1.67 in this analysis.
Effects of Admission SAT on Outcomes
There was no significant association between SAT and survival at 6 months (OR, 0.99; 95% CI, 0.997-1.001; P = .32), likelihood of in-hospital death (OR, 0.99; 95% CI, 0.998-1.001; P = .32), disability at hospital discharge (P = .40), or ICU-free days to day 28 (slope, -0.001 ± 0.003; r2 = 0.04; P = .68) (Table 2). The lack of significant associations persisted even after incorporating SAT in the multivariable modeling for 6-month mortality (P = .785) (Table 3) and the other outcomes measures (e-Tables 6-8).
The Effect of Sex on the Association of PMA With Outcomes
A post hoc analysis indicated that the association of PMA with outcomes was consistent between sexes: larger muscle mass was associated with better outcomes. However, there were differences of magnitude depending on the sex: PMA association with ICU-free days appeared more robust in female patients than in male patients (Table 4) despite no significant differences in baseline clinical characteristics, except for a larger SAT in female patients (e-Table 9, Table 5). This post hoc analysis also suggested that smaller muscle mass may be associated with higher odds of disability at discharge in male patients but not in the overall cohort (Table 2). The described results used the correction factor for female muscle size.21, 22 However, whether using the female correction factor, correcting for ideal body weight,23 or not using any correction method, the effects of PMA over 6-month survival, ICU-free days, and hospital survival remained statistically significant; and the effect of PMA over disability at hospital discharge remained nonsignificant (e-Table 10).
Table 4.
Univariate Analysis of PMA Associations With Outcomes According to Patient Sex
| Sex | ICU-Free Days to Day 28a,b | Disability at Dischargeb,c | Hospital Mortalityb,d | 6-Month Survival |
|---|---|---|---|---|
| Male | Slope, 0.017 ± 0.025; P = .5 | OR, 0.65 (0.53-0.80); P < .001 | OR, 0.96 (0.93-0.99); P = .002 | OR, 1.04 (1.02-1.07); P ≤ .001 |
| Female | Slope, 0.073 ± 0.029; P = .012 | OR, 0.87 (0.73-1.04); P = .09 | OR, 0.95 (0.92-0.98); P < .001 | OR, 1.01 (0.99-1.03); P = .09 |
See Table 1 legend for expansion of abbreviation.
ICU-free days to day 28 reached significance in female subjects and not in male subjects.
Outcome per square centimeters of PMA.
Disability at hospital discharge reached significance in male subjects and not in female subjects.
Hospital mortality reached significance in both sexes.
Table 5.
Baseline Characteristics According to Patient Sex
| Characteristic | Male | IQR | Female | IQR | P Value |
|---|---|---|---|---|---|
| Demographic | |||||
| Age | 59.76 | 51-69 | 61.31 | 49-75 | .32 |
| Raw PMA | 42.7 | 33.2-54-8 | 27.05 | 21.8-33.8 | < .001 |
| Corrected PMA | 42.7 | 33.2-54-8 | 45.13 | 36.4-56.4 | .783 |
| SAT | 17.8 | 10.1-23.8 | 23.1 | 13.2-30.7 | < .001 |
| Severity scores | |||||
| APACHE II | 16.99 | 11-22 | 16 | 11-22 | .73 |
| SOFA | 5.26 | 3-7 | 5.25 | 3-7 | .98 |
| Male | % | Female | % | P Value | |
|---|---|---|---|---|---|
| Preadmission comorbidities | |||||
| Pulmonary disease | 83 | 36.8 | 74 | 41.3 | .36 |
| Steroid use | 52 | 23 | 52 | 29 | .17 |
| Diabetes mellitus | 60 | 26.6 | 41 | 23 | .38 |
| Cancer | 50 | 22 | 40 | 22 | .95 |
| Congestive heart failure | 47 | 20 | 30 | 17 | .29 |
| End-stage renal disease | 24 | 10 | 9 | 5 | .06 |
| Preadmission mMRC | 2 | 1-4* | 2 | 1-4* | .65 |
| Male | % | Female | % | P Value | |
|---|---|---|---|---|---|
| Primary reason of ICU admission | |||||
| Respiratory failure | 92 | 41 | 79 | 44 | .51 |
| Nonrespiratory sepsis | 38 | 16.8 | 33 | 18.4 | .34 |
| Pulmonary embolism | 36 | 16 | 30 | 16.7 | .42 |
| Hemorrhagic shock | 14 | 6.2 | 8 | 4.4 | .22 |
| Altered mental status | 10 | 4.4 | 11 | 6 | .22 |
| Metabolic cause, including diabetic ketoacidosis | 9 | 4 | 4 | 4.3 | .16 |
| Trauma | 7 | 3.1 | 4 | 2.2 | .29 |
| Cardiovascular decompensation | 10 | 4.4 | 5 | 2.7 | .19 |
| Other | 6 | 2.6 | 3 | 3 | .34 |
IQR = interquartile range. See Table 1 legend for expansion of other abbreviations.
Discussion
In the study cohort of 401 patients admitted to an ICU, we report a significant association of larger admission PMA with higher 6-month and hospital survival and more ICU-free days. This effect on survival persisted even after adjusting for other variables, including severity score, admission albumin and subcutaneous fat, and exercise limitation represented by the modified Medical Research Council (mMRC) score. Consistent with the fact that pectoralis muscles are not involved in locomotion, PMA was unable to predict, among the survivors, the regaining of independent life at discharge vs the need for assisted living. We believe that PMA likely reflects the general health status24 and is a marker of prehospitalization frailty.25, 26
A post hoc analysis of our data suggests that the association of PMA with ICU-free days was statistically significant in female patients but not in male patients; also, smaller PMA predicted a greater likelihood of disability at hospital discharge only in male patients. We found no obvious cause for this finding, including prehospital morbidities, preadmission albumin, mMRC score, age, severity of disease, or reason for admission to the ICU; however, SAT was larger in female patients. Although these conclusions are necessarily speculative given the post hoc approach, future research specifically designed to assess sex-specific effects on muscle turnover in the context of critical illness could potentially add insights concerning our findings. Indeed, although the present research did not address the mechanisms of muscle wasting in critical illness, other data revealed fewer muscle satellite (stem) cells in ICU survivors,7 and these cells have been found to work more efficiently in female animals than in male animals,27 which suggests that female subjects could possibly have different muscle regeneration capacity compared with male subjects during and after critical illness.
To our knowledge, this analysis is the first large prospective study that has investigated the association of skeletal muscle mass at the time of ICU admission with survival. Previously, a retrospective study focused on elderly ICU patients found an association of admission lumbar muscle mass with ICU-free days and mortality.28 However, sarcopenia, the muscle loss associated with advanced age,29, 30 is a distinct entity that cannot be extrapolated to younger populations.31, 32 Other retrospective data also indicated that peri-admission lower lumbar muscle area is associated with ICU mortality and with discharge to a nursing home.15 Because the muscle measurements in that study had taken place up to 4 days following ICU admission,15 it is conceivable that it aggregated a significant percentage of patients who developed muscle wasting during their ICU stay with those who had muscle wasting prior to the admission. Indeed, there is consistent evidence that accelerated muscle wasting occurs early in the ICU course.1 It is also possible that the association of PMA with ICU outcomes is different from other muscle groups’ similarly to the heterogeneous effects of diverse muscle areas on COPD prognosis.2
The present analysis was also the first to explore the association of fat mass at the time of ICU admission with survival, and no significant correlations were found. Although previous evidence indicates that higher preadmission weight associates with better post-ICU status,16 it is unclear which body compartment accounted for the weight’s salutatory effect in that study. Indeed, it has been reported that obesity associates with greater survival in critical illness33 and that extreme obesity is not associated with worse survival advantage than normal weight.34 Thus, it is plausible that fat, and not muscle, contributes to better outcomes reflected by greater weight. Although our data do not necessarily challenge the beneficial35 or harmful36 effects of obesity on ICU prognosis, we found that admission SAT measured at the T7-T8 level had no association with all of the impactful ICU outcomes that we explored a priori, whereas PMA was associated with several of these outcomes, including survival.
The persistence of the association of muscle wasting with mortality after adjusting for other covariates suggests a potential benefit for incorporating muscle mass into scoring systems used to predict ICU outcomes, particularly with the development of standardized bedside muscle ultrasound techniques.37, 38 These data could also stimulate interest in determining if muscle-survival association portends causality39, 40 and whether preventing or reversing muscle wasting potentially entails mortality benefits in critical illness. Indeed, the present data suggest that optimizing muscle status in patients at risk of developing critical illness such those planning to undergo major surgery may improve outcomes.
The main strengths of the present study are the large size of the study cohort and the prospective study design. In addition, the selection of the pectoralis muscle, which is easily measurable41 and not necessarily associated with a specific functional domain such as locomotion or ventilation, allowed us to assess the systemic/global effects of muscle wasting on ICU outcomes. Furthermore, pectoralis muscles have both type I (oxidative) and type II (glycolytic) fibers,42 and thus PMA assessment reflects muscle wasting even in the presence of comorbidities that exhibit selective fiber-type atrophy.43 Our study has some limitations. First, it was performed at a single institution, and although the admission diagnoses of the study cohort were diverse, it is possible that the distribution of diagnoses is not generalizable to other ICUs. Second, chest CT scans were performed for a clinical indication and not as part of a study protocol. Because certain diagnoses are more frequently evaluated by using chest CT scan than others, there may have been a significant selection bias in the cohort. However, this bias is unlikely to significantly compromise the generalization of the current findings as the study cohort mirrored the characteristics of patients analyzed in a systematic review of high-quality, prospective data involving 31 ICU studies and 3,905 patients,44 in which 39% of ICU admissions were due to respiratory failure (42% in the present cohort); 15% were due to sepsis (17% in the present cohort); and average age in these studies was 61 years (62 years in the present cohort). Third, the use of the PMA surrogate of muscle mass in ICU patients has not been validated, although accumulating literature describes its use in healthy41 and chronically ill18, 45 patients. Likewise, although SAT measurement at the T7-T8 level has not been validated for ICU patients, it has been shown to be highly correlated with the thoracic adipose tissue volume and with BMI.19 Fourth, because patients were enrolled after they had already developed critical illness, we had to select a surrogate measure of prehospitalization mobility status. Although previous research has used dichotomized surrogates of preadmission exercise capacity such as the ability to ambulate up 10 stairs before hospitalization,46 we chose the mMRC score because of its simplicity, very high interobserver agreement, and adequate correlation with other scoring systems.47 We realize that the mMRC is potentially confounded by cardiopulmonary limitations and other factors. However, as many of our patients were unable to cooperate with the evaluation, volitional tests such as the MRC muscle strength scoring system or other direct muscle evaluations were not feasible.48 Fifth, muscle dysfunction effect on disability at hospital discharge could potentially be influenced by sedation or mobilization protocols, which we could not correct for given the strong collinearity with days in the ICU and receiving mechanical ventilation, both incorporated to the ICU-free days to day 28. Future studies powered for these corrections could determine the interaction between baseline muscle mass, disability at hospital discharge, sedation, and mobilization protocols.
Conclusions
These data show that admission muscle mass of ICU patients is associated with 6-month survival, ICU-free days, and hospital survival. Because all these outcomes are highly relevant, we postulate that further investigation of muscle turnover in the ICU patient may clarify whether better muscle mass can lead to improved outcomes. Preadmission muscle mass may be a useful biomarker for important ICU outcomes. SAT was not associated with disability and survival during and following critical illness.
Acknowledgments
Author contributions: A. J. and M. H. S. K. screened eligible patients, and M. H. S. K., R. I., and H. C. C. enrolled the patients. P. N. made the PMA measurements, and C. L. D. made the SAT measurements on the CT scans; J. P. F. confirmed the measurements. R. M. Y. performed the preliminary statistical analyses, and P. J. F. performed the final statistical analyses. A. J., R. M. Y., P. J. F., and M. A. J. designed the trial, and A. J. and M. A. J. wrote and edited the paper.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: M. A. J. has been a consultant for Biogren and aTyr Pharma. None declared (A. J., M. M. H. S. K., R. I., H. C., C. D., P. N., J. F., R. Y., P. J. F.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: The authors thank Kanwaldeep Williams, MBA, for the diligent work in providing data of the nonenrolled ICU patients’ census; Donna A. R. Kriegbaum, MS, RD, CNSC, for the advice on the nutritional evaluation of the enrolled patients; and physicians Mariana I. Labato, MD, and Esther Barreiro, MD, PhD, for the critical reading of the manuscript.
Additional information: The e-Appendix, e-Figures, and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: Part of the results reported here were funded by the National Heart, Lung, and Blood Institute of the National Institutes of Health [Grant K01-HL130704 to A. J.], and by the Collins Family Foundation Endowment [to A. J.].
Supplementary Data
References
- 1.Puthucheary Z.A. Acute skeletal muscle wasting in critical illness. JAMA. 2013;310:1591–1600. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 2.Jaitovich A., Barreiro E. Skeletal muscle dysfunction in chronic obstructive pulmonary disease (COPD): what we know and can do for our patients. Am J Respir Crit Care Med. 2018;198:175–186. doi: 10.1164/rccm.201710-2140CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali N.A. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med. 2008;178:261–268. doi: 10.1164/rccm.200712-1829OC. [DOI] [PubMed] [Google Scholar]
- 4.Sharshar T. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med. 2009;37:3047–3053. doi: 10.1097/CCM.0b013e3181b027e9. [DOI] [PubMed] [Google Scholar]
- 5.De Jonghe B. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med. 2007;35:2007–2015. doi: 10.1097/01.ccm.0000281450.01881.d8. [DOI] [PubMed] [Google Scholar]
- 6.Adler D., Dupuis-Lozeron E., Richard J.C., Janssens J.P., Brochard L. Does inspiratory muscle dysfunction predict readmission after intensive care unit discharge? Am J Respir Crit Care Med. 2014;190:347–350. doi: 10.1164/rccm.201404-0655LE. [DOI] [PubMed] [Google Scholar]
- 7.Dos Santos C. Mechanisms of chronic muscle wasting and dysfunction after an intensive care unit stay. A pilot study. Am J Respir Crit Care Med. 2016;194:821–830. doi: 10.1164/rccm.201512-2344OC. [DOI] [PubMed] [Google Scholar]
- 8.Herridge M.S. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 9.Chan K.S. Evaluating muscle mass in survivors of acute respiratory distress syndrome: a 1-year multicenter longitudinal study. Crit Care Med. 2018;46:1238–1246. doi: 10.1097/CCM.0000000000003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberda C. The relationship between nutritional intake and clinical outcomes in critically ill patients: results of an international multicenter observational study. Intensive Care Med. 2009;35:1728–1737. doi: 10.1007/s00134-009-1567-4. [DOI] [PubMed] [Google Scholar]
- 11.Kelmenson D.A. Outcomes of ICU patients with a discharge diagnosis of critical illness polyneuromyopathy: a propensity-matched analysis. Crit Care Med. 2017;45:2055–2060. doi: 10.1097/CCM.0000000000002763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goligher E.C. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med. 2018;197:204–213. doi: 10.1164/rccm.201703-0536OC. [DOI] [PubMed] [Google Scholar]
- 13.Herridge M.S. The RECOVER program: disability risk groups and 1-year outcome after 7 or more days of mechanical ventilation. Am J Respir Crit Care Med. 2016;194:831–844. doi: 10.1164/rccm.201512-2343OC. [DOI] [PubMed] [Google Scholar]
- 14.Looijaard W.G. Skeletal muscle quality as assessed by CT-derived skeletal muscle density is associated with 6-month mortality in mechanically ventilated critically ill patients. Crit Care. 2016;20:386. doi: 10.1186/s13054-016-1563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weijs P.J. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care. 2014;18:R12. doi: 10.1186/cc13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrante L.E. Factors associated with functional recovery among older intensive care unit survivors. Am J Respir Crit Care Med. 2016;194:299–307. doi: 10.1164/rccm.201506-1256OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaitovich A. ICU admission skeletal muscle mass, in-hospital outcomes and 6-months mortality: a prospective study. Am J Respir Crit Care Med. 2017;195:A2775. [Google Scholar]
- 18.McDonald M.L. Quantitative computed tomography measures of pectoralis muscle area and disease severity in chronic obstructive pulmonary disease. A cross-sectional study. Ann Am Thorac Soc. 2014;11:326–334. doi: 10.1513/AnnalsATS.201307-229OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong Y. Chest fat quantification via CT based on standardized anatomy space in adult lung transplant candidates. PLoS One. 2017;12:e0168932. doi: 10.1371/journal.pone.0168932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young P. Acetaminophen for fever in critically ill patients with suspected infection. N Engl J Med. 2015;373:2215–2224. doi: 10.1056/NEJMoa1508375. [DOI] [PubMed] [Google Scholar]
- 21.Fuchs G. Lumbar skeletal muscle index derived from routine computed tomography exams predict adverse post-extubation outcomes in critically ill patients. J Crit Care. 2018;44:117–123. doi: 10.1016/j.jcrc.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 22.Janssen I., Heymsfield S.B., Wang Z.M., Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18-88 yr. J Appl Physiol (1985) 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 23.Pai M.P., Paloucek F.P. The origin of the “ideal” body weight equations. Ann Pharmacother. 2000;34:1066–1069. doi: 10.1345/aph.19381. [DOI] [PubMed] [Google Scholar]
- 24.Casaer M.P., Van den Berghe G. Nutrition in the acute phase of critical illness. N Engl J Med. 2014;370:1227–1236. doi: 10.1056/NEJMra1304623. [DOI] [PubMed] [Google Scholar]
- 25.Ferrante L.E. Functional trajectories among older persons before and after critical illness. JAMA Intern Med. 2015;175:523–529. doi: 10.1001/jamainternmed.2014.7889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrante L.E. The association of frailty with post-ICU disability, nursing home admission, and mortality: a longitudinal study. Chest. 2018;153:1378–1386. doi: 10.1016/j.chest.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deasy B.M. A role for cell sex in stem cell-mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. J Cell Biol. 2007;177:73–86. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moisey L.L. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care. 2013;17:R206. doi: 10.1186/cc12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Evans W.J. What is sarcopenia? J Gerontol A Biol Sci Med Sci. 1995;50(Spec No):5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- 30.Hepple R.T. Muscle atrophy is not always sarcopenia. J Appl Physiol (1985) 2012;113:677–679. doi: 10.1152/japplphysiol.00304.2012. [DOI] [PubMed] [Google Scholar]
- 31.Delbono O. Expression and regulation of excitation-contraction coupling proteins in aging skeletal muscle. Curr Aging Sci. 2011;4:248–259. doi: 10.2174/1874609811104030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sandri M. Signalling pathways regulating muscle mass in ageing skeletal muscle: the role of the IGF1-Akt-mTOR-FoxO pathway. Biogerontology. 2013;14:303–323. doi: 10.1007/s10522-013-9432-9. [DOI] [PubMed] [Google Scholar]
- 33.O'Brien J.M., Jr. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med. 2006;34:738–744. doi: 10.1097/01.CCM.0000202207.87891.FC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martino J.L. Extreme obesity and outcomes in critically ill patients. Chest. 2011;140:1198–1206. doi: 10.1378/chest.10-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shashaty M.G., Stapleton R.D. Physiological and management implications of obesity in critical illness. Ann Am Thorac Soc. 2014;11:1286–1297. doi: 10.1513/AnnalsATS.201404-159FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paolini J.B. Predictive value of abdominal obesity vs. body mass index for determining risk of intensive care unit mortality. Crit Care Med. 2010;38:1308–1314. doi: 10.1097/CCM.0b013e3181d8cd8b. [DOI] [PubMed] [Google Scholar]
- 37.Puthucheary Z.A. Rectus femoris cross-sectional area and muscle layer thickness: comparative markers of muscle wasting and weakness. Am J Respir Crit Care Med. 2017;195:136–138. doi: 10.1164/rccm.201604-0875LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Puthucheary Z.A. Qualitative ultrasound in acute critical illness muscle wasting. Crit Care Med. 2015;43:1603–1611. doi: 10.1097/CCM.0000000000001016. [DOI] [PubMed] [Google Scholar]
- 39.Files D.C. A critical role for muscle ring finger-1 in acute lung injury-associated skeletal muscle wasting. Am J Respir Crit Care Med. 2012;185:825–834. doi: 10.1164/rccm.201106-1150OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaitovich A. High CO2 levels cause skeletal muscle atrophy via AMP-activated kinase (AMPK), FoxO3a protein, and muscle-specific Ring finger protein 1 (MuRF1) J Biol Chem. 2015;290:9183–9194. doi: 10.1074/jbc.M114.625715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim Y.S., Kim E.Y., Kang S.M., Ahn H.K., Kim H.S. Single cross-sectional area of pectoralis muscle by computed tomography—correlation with bioelectrical impedance based skeletal muscle mass in healthy subjects. Clin Physiol Funct Imaging. 2017;37:507–511. doi: 10.1111/cpf.12333. [DOI] [PubMed] [Google Scholar]
- 42.Gokhin D.S. Thin-filament length correlates with fiber type in human skeletal muscle. Am J Physiol Cell Physiol. 2012;302:C555–C565. doi: 10.1152/ajpcell.00299.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ciciliot S., Rossi A.C., Dyar K.A., Blaauw B., Schiaffino S. Muscle type and fiber type specificity in muscle wasting. Int J Biochem Cell Biol. 2013;45:2191–2199. doi: 10.1016/j.biocel.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 44.Fan E. An official American Thoracic Society Clinical Practice guideline: the diagnosis of intensive care unit-acquired weakness in adults. Am J Respir Crit Care Med. 2014;190:1437–1446. doi: 10.1164/rccm.201411-2011ST. [DOI] [PubMed] [Google Scholar]
- 45.Kinsey C.M. Lower pectoralis muscle area is associated with a worse overall survival in non-small cell lung cancer. Cancer Epidemiol Biomarkers Prev. 2017;26:38–43. doi: 10.1158/1055-9965.EPI-15-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Detsky M.E. Discriminative accuracy of physician and nurse predictions for survival and functional outcomes 6 months after an ICU admission. JAMA. 2017;317:2187–2195. doi: 10.1001/jama.2017.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mahler D.A., Wells C.K. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93:580–586. doi: 10.1378/chest.93.3.580. [DOI] [PubMed] [Google Scholar]
- 48.Hough C.L., Lieu B.K., Caldwell E.S. Manual muscle strength testing of critically ill patients: feasibility and interobserver agreement. Crit Care. 2011;15:R43. doi: 10.1186/cc10005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.