Abstract
Purpose:
Despite extensive knowledge gained over the last three decades regarding limbal stem cell deficiency (LSCD), the disease is not clearly defined, and there is lack of agreement on the diagnostic criteria, staging and classification system among treating physicians and research scientists working on this field. There is therefore an unmet need to obtain global consensus on the definition, classification, diagnosis and staging of LSCD.
Methods:
A Limbal Stem Cell Working Group was first established by the Cornea Society in 2012. The Working Group was divided into subcommittees. Four face-to-face meetings, frequent email discussions and teleconferences were conducted since then to obtain agreement on a strategic plan and methodology from all the participants after a comprehensive literature search, and reached final agreement on the definition, classification, diagnosis and staging of LSCD. A writing group was formed to draft the current manuscript, which has been extensively revised to reflect the consensus of the Working Group.
Results:
A consensus was reached on the definition, classification, diagnosis and staging of LSCD. The clinical presentation and diagnostic criteria of LSCD were clarified, and a staging system of LSCD based on clinical presentation was established.
Conclusions:
This global consensus provides a comprehensive framework for the definition, classification, diagnosis and staging of LSCD. The newly established criteria will aid in the correct diagnosis and formulation of an appropriate treatment for different stages of LSCD that will facilitate a better understanding of the condition, help with clinical management, research and clinical trials in this area.
Keywords: limbal stem cell deficiency, limbal stem cells, conjunctiva, cornea, definition, classification, diagnosis, markers, impression cytology, anterior segment optical coherence tomography, in vivo confocal microscopy
Summary
Limbal stem cell deficiency can be caused by genetic defect(s) or secondary insult to LSCs and their niche, which can result in abnormal corneal epithelial wound healing and/or conjunctivalization of the corneal surface. Late corneal fluorescein staining, recurrent or persistent epithelial defect, and epithelial thinning with or without vascularization while frequently seen in LSCD, are not unique to LSCD. The presence of one or more of these signs might not be sufficient to make the diagnosis. The clinical presentation of LSCD varies based on the severity. The severity of LSCD is classified into three defined stages based on clinical presentation. Diagnostic tests include detection of conjunctival cells using impression cytology, IVCM, and AS-OCT of the cornea and limbus. Because of the limitations of clinical examination, a diagnostic test is recommended, if available, to confirm the diagnosis of LSCD before any surgical invention. This global consensus statement provides a comprehensive framework for the definition, classification, staging and diagnosis of LSCD.
Introduction
Normal function of the epithelial stem cells at the corneoscleral limbus is a prerequisite for the maintenance and regeneration of the corneal epithelium. Although there is disagreement on which marker(s) is specific for corneal epithelial stem cells, it is generally believed that they reside in the basal epithelium of the limbus, which is the transition zone between the transparent cornea and the opaque sclera.1 For this reason, these stem cells are referred to as limbal epithelial stem cells (LSCs). The normal limbus and LSCs act as a barrier against the invasion of conjunctival epithelium onto the corneal surface.2
Evidence for the location of stem cells at the limbus originates mainly from studies of their proliferation and differentiation. Within the limbal basal epithelium exists a population of cells that are normally quiescent (slow-cycling).3 These cells lack the differentiation features of the corneal epithelium and are the most undifferentiated cells within the corneolimbal epithelium.1 Furthermore, these cells express numerous markers that are characteristic of stem and progenitor cells in other tissues.4,5
One key characteristic of stem cells is the tight regulation of their state of differentiation and proliferation by the microenvironment in their immediate neighborhood, which is commonly referred to as the stem cell niche.6,7 In the human eye the palisades of Vogt (POV) with the underlying limbal vasculature function as a niche for LSCs.8 In addition to the stem and progenitor cells organized in clusters, the limbal niche contains several types of supporting cells (niche cells), a specific extracellular matrix, and soluble factors mediating biochemical and biophysical signals.9,10 An orchestrated interaction of the various factors within the niche is a prerequisite for the undisturbed function of LSCs to keep a stable ocular surface.11–13 Therefore, the disturbance of the niche in the context of disease, injuries, and uncontrolled inflammation will lead to aplasia and depletion of LSCs; subsequently the ability to sustain the regeneration of the corneal epithelium is lost. Therapeutic strategies to repair LSC function not only must replenish the stem cell population but also attempt to restore the stem cell niche.
The important role of LSCs in the maintenance of the normal homeostasis of corneal epithelium and wound healing has been confirmed in a variety of experimental animal models.14–16 In vivo evidence has demonstrated that a certain number of LSCs is necessary to maintain a healthy corneal epithelium.15,17,18 Based on the concept of LSCs, Kenyon and Tseng demonstrated in 1989 that the corneal epithelial phenotype can be reconstituted in patients with unilateral limbal stem cell deficiency (LSCD) by transplantation of autologous corneal epithelial stem cells from the contralateral healthy eye.19 For bilateral disease, allograft transplantation was later introduced with much lower success rates, primarily because of immunologic rejection.17,20 Cultivated autologous LSCs were subsequently developed to treat patients with LSCD.21,22
The establishment and application of the concept of LSCs have led to extraordinary progress in the development of procedures and cell-based therapies for ocular surface rehabilitation, benefiting patients tremendously over the last 3 decades. The multitude of surgical approaches of transplanting LSCs onto diseased eyes has led to a classification system for ocular surface rehabilitation procedures developed by the Cornea Society. The purposes of this classification system are to improve the clarity of communication and to enable the comparison of outcomes of these innovative procedures.23
In contrast to the surgical treatment of LSCD, there is no agreement on the disease entity of LSCD. Since the introduction of the LSC concept in 1980s’, numerous reports have used a wide variety of terms to describe LSCD and have provided various definitions of this disease. Currently, there is neither a generally accepted classification nor a staging system for this disease entity. Especially in the context of therapeutic strategies, numerous diagnostic approaches have been described to identify LSCD, and the variety in these approaches makes it impossible to compare the results of clinical trials. The Cornea Society realized that there was a need for an international agreement about the definition, classification, diagnosis and staging of LSCD.
Methods
The Cornea Society formed the Limbal Stem Cell Working Group in 2012 to obtain a consensus from a group of experts in the field of LSCD. The working group consisted of members of the Cornea Society, the Asian Cornea Society, PanCornea, and EU-Cornea; these members were experts on cornea and external diseases with extensive experience in the field of LSCD, had been authors of high-impact scientific publications, were recognized internationally by the cornea community, and were willing to attend face-to-face meetings and comply with project timelines. A subcommittee was established to devise a proposed definition, classification system, and staging and diagnostic criteria for LSCD. Literature searches were performed by members who were assigned topics; their aim was to identify criteria reported for which inclusion in the new consensus would be appropriate. The working group met face to face on 4 occasions between 2012 to 2013 to formulate a strategic plan and methodology from all the participants and discuss the definition, classification system, and diagnostic and staging criteria. Subsequently, frequent email discussions and multiple teleconferences occurred to further refine the criteria. Members who attended 2 or more of the meetings are listed in Acknowledgments. Once there was a broad consensus on all topics, a writing committee was formed. The results were summarized by the writing committee at the final meeting in 2017. The draft of the manuscript was circulated for further comments. The final draft was then approved by all members of the writing committee of this manuscript.
Results
Definition of limbal stem cell deficiency
Limbal stem cell deficiency is an ocular surface disease caused by a decrease in the population and/or function of corneal epithelial stem/progenitor cells; this decrease leads to the inability to sustain the normal homeostasis of the corneal epithelium. The disease is characterized by conjunctivalization (i.e., replacement of the normal corneal epithelium by conjunctival epithelium) and/or other signs of epithelial dysfunction such as persistent or recurrent epithelial defects with or without neovascularization, ocular surface inflammation, and scarring. Frequent consequences are decreased vision and discomfort leading to reduced health-related quality of life (HRQOL). Limbal stem cell deficiency may present alone as a single entity or associated with abnormalities of other components of the ocular surface such as the conjunctiva, meibomian glands, lacrimal glands, tears, corneal nerves and immune system.
Partial LSCD is characterized by incomplete conjunctivalization of the corneal surface and the presence of residual limbal and consequent corneal epithelial cells (Figure 1C, 1D). Total LSCD is characterized by conjunctivalization of the entire corneal surface due to a complete loss of corneal epithelial stem/progenitor cells (Figure 1E, 1F).
Figure 1.
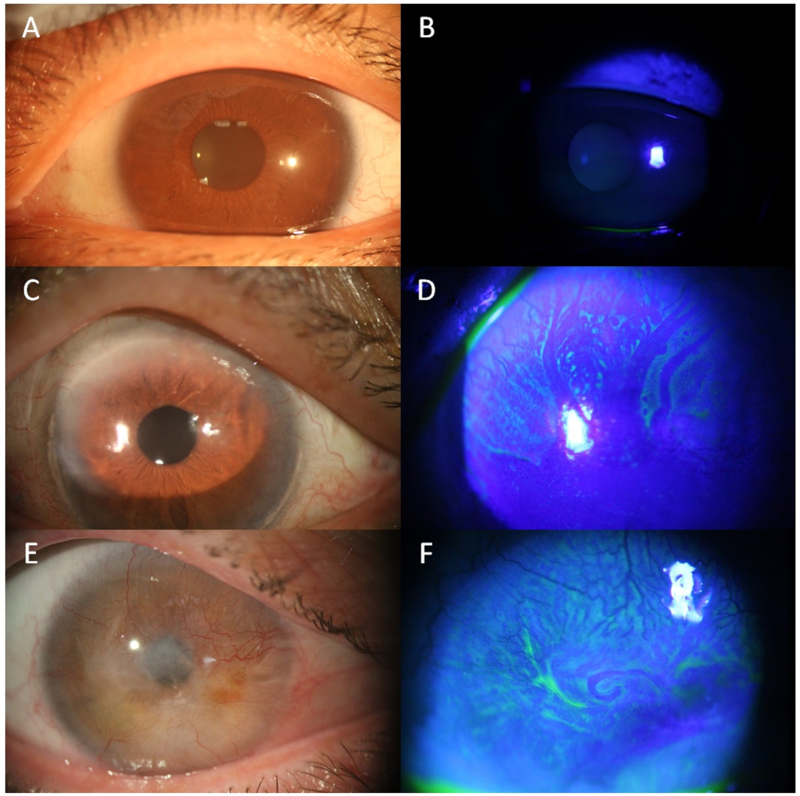
Slit lamp photos (bright light and fluorescein staining). Slit lamp photos of normal eyes (A,B), sectoral limbal stem cell defciency (LSCD, C,D) and total LSCD eyes (E, F). The epithelium in the normal eye is transparent (A), which cannot be stained by fluorescein (B). In the eye with sectoral LSCD, the epithelium of affected area superiorly is greyish (C) under bright light. It can be better visualized with fluorescein staining under cobalt blue light, which often have a whorl-like pattern and fluorescein pooling in area where there is epithelial thinning (D). In eyes with total LSCD, corneal opacity and superficial neovascularization affecting the entire cornea sruface are visible under bright light (E). Fluorescein staining shows epithelial irregularity (F).
Classification of limbal stem cell deficiency
Limbal stem cell deficiency is due to a variety of causes (Table 1). The cause of LSCD in some cases are unknown (idiopathic LSCD).
Table 1.
Etiology of limbal stem cell deficiency.
| Acquired limbal stem cell deficiency |
| Acquired nonimmune-mediated |
| Chemical injury27,42 |
| Thermal injury42 |
| Radiation injury45 |
| Contact lens wear28,42 |
| Multiple surgeries involving the limbus30,42 |
| Bullous keratopathy40,41 |
| Infectious ocular disease28,43,71 |
| Chronic lid disease |
| Severe blepharitis-rosacea43 |
| Trachoma42 |
| Tumors of the ocular surface44 |
| Severe pterygium29,30 |
| Drug induced LSCD |
| Mitomycin C33–35 |
| 5-fluorouracil31 |
| Preservative36 |
| Systemic chemotherapy and immunotherapy37–39 |
| Acquired primary immune-mediated |
| Stevens-Johnson syndrome/toxic necrolysis17,42,46,47 |
| mucous membrane pemphigoid 50,51 |
| Allergic ocular surface disease |
| Vernal keratoconjunctivitis53,54 |
| Atopic keratoconjunctivitis56,57 |
| Graft-vs-host disease58,59 |
| Idiopathic |
| Hereditary limbal stem cell deficiency |
| Congenital aniridia43,71 |
| Dyskeratosis congentia 65 |
| Autoimmune polyendocrinopathy-candidiasis–ectodermal dystrophy/dysplasia (Multiple endocrine deficiency, APS1)62,63 |
| Xeroderma pigmentosum64 |
| Keratitis ichthyosis deafness syndrome68,69 |
| Ectrodactyly-ectodermal dysplasia-clefting syndrome66,67 |
| Lacrimo-auriculo-dental-digital syndrome70 |
| Epidermolysis bullosa71,72 |
1. Acquired limbal stem cell deficiency
1.1. Acquired nonimmune-mediated limbal stem cell deficiency
1.1.1. Chemical and thermal injury
Whereas data regarding the epidemiology of LSCD are not available, a reported series of patients with LSCD showed that the leading cause of LSCD is ocular burn: at least two-thirds of cases of LSCD that had undergone LSC transplantation are due to ocular surface burn (Suppl Table 1). Chemical ocular injuries in general appear to be more common in males than females. 24 Chemical and thermal injuries can result in the destruction of LSCs and their niche. At the acute stage of ocular burns, evidence of more than 50% of limbal ischemia is a strong risk factor for the development of LSCD. Among chemical injuries, alkali burns appear to be more common and result in more severe tissue damage than acid burns as alkali chemicals induce saponification of cellular membranes with deeper penetration in the limbal tissue, potentially destroying the limbal niche and LSCs.25,26 Sulfur mustard, which is a potent chemical warfare vesicating agent can result in delayed-onset keratopathy and LSCD.27 Prolonged ocular surface inflammation and denervation after chemical injury may cause further damage to the residual LSCs. Thermal burns appear to be much less frequent than chemical injuries (Suppl Table 1).
1.1.2. Contact lens wear
The term contact lens–induced keratopathy has been used to describe the clinical presentation of LSCD associated with contact lens wear. The exact pathophysiology of contact lens–induced LSCD28 is unclear, but it is probably multifactorial as a result of poor contact lens fitting, low oxygen permeability of the contact lens material, prolonged/extended wear, and sensitivity of some contact lens wearers to the toxicity of contact lens cleaning and storage solutions. Most cases of contact lens–induced LSCD are reversible after the discontinuation of contact lens wear and medical treatment.
1.1.3. Surgery
Eye surgery with limbal involvement, including the excision of limbal and conjunctival tumors, repeated and extensive pterygium surgery,29,30 and trabeculectomy with the use of anti-metabolites31–33 can induce LSCD as a result of the direct destruction of LSCs and the limbal niche. The extent of LSCD is often sectoral. The term iatrogenic LSCD has been used to categorize LSCD caused by ocular surgeries and medications.30
1.1.4. Toxicity from medications
Both topical drugs, in particular, antimetabolites mitomycin C33–35 and 5-fluorouracil;31 preservatives in eye medications;36 and systemic chemotherapies (hydroxyurea,37 S-1,38 hydroxycarbamide39) have been reported as causes of LSCD.
1.1.5. Bullous keratopathy
Long-standing, advanced bullous keratopathy could cause LSCD, and squamous metaplasia is associated with LSCD in eyes with this type of keratopathy.40,41 After successful corneal transplantation, LSCD may improve in affected eyes.
1.1.6. Other
Limbal stem cell deficiency is considered as a part of the pathophysiology of pterygium, and excision of extensive pterygium may also cause LSCD. Severe microbial keratitis including trachoma could lead to LSCD.42,43 Potential mechanisms for LSC destruction by microbial keratitis include severe inflammation of the ocular surface, infectious agent-induced LSC necrosis, toxicity of the anti-microbial medications, and progressive fibrosis of the ocular surface following the acute stage of infection. In the case of trachoma, chronic microtrauma to the corneal surface by the eyelid abnormality contributes to the pathogenesis of LSCD.
Other possible causes of LSCD include severe chronic rosacea blepharoconjunctivitis often in the setting of other ocular surface diseases, advanced ocular surface squamous cell carcinoma, 44 and radiation.45 These etiologies of LSCD each represent less than 5% of all etiologies.
1.2. Acquired primary immune-mediated LSCD
1.2.1. Stevens-Johnson syndrome/toxic epidermal necrolysis spectrum disease
Stevens-Johnson syndrome (SJS)/toxic epidermal necrolysis (TEN) spectrum disease is a rare hypersensitivity reaction that typically involves the skin and various mucous membranes including the ocular surface. The most severe ocular outcome in SJS/TEN spectrum disease is the destruction and resultant aplasia of the LSCs.17,42,46 Primary corneal involvement in SJS is not common; LSCD more commonly results from secondary corneal involvement due to chronic microtrauma and persistent ocular surface inflammation.47 This microtrauma may occur in the context of pre-existing structural anomalies of eyelid position, trichiasis, or symblepharon. Moreover, scarring of lacrimal duct orifices and the destruction of conjunctival goblet cells and meibomian gland orifices may lead to a combination of severe aqueous, mucin, and lipid tear deficiencies, exacerbating LSCD.46,48,49
1.2.2. Mucous membrane pemphigoid (MMP)
This group of chronic inflammatory, subepithelial, blistering diseases predominantly affects the mucous membranes through the deposition of IgG, IgA, and/or C3. Ocular involvement in mucous membrane pemphigoid (ocular MMP, formerly referred to as ocular cicatricial pemphigoid) is characterized by chronic or relapsing inflammation and subconjunctival fibrosis, followed by fornix foreshortening and obliteration, symblepharon formation, and, in severe cases, ankyloblepharon.50,51 Scarring of lacrimal duct orifices may lead to severe dry eye, which exacerbates LSC dysfunction. The alterations of the adnexa and the blink-related microtrauma exacerbate inflammation in the ocular surface unit, including limbus, weakening stromal support and leading to the gradual loss of LSCs.52
1.2.3. Allergic ocular surface diseases
1.2.3.1. Vernal keratoconjunctivitis
The palpebral and limbal forms of vernal keratoconjunctivitis (VKC) is sometimes associated with LSCD.53,54 In the palpebral form, fibroblast activation and collagen hyperproduction lead to giant papillary hypertrophy with associated lid abnormalities and poor ocular surface conditions. In the limbal form, alterations of the extracellular matrix molecules caused by inflammatory insults erode the stromal support of the limbal niche. Eosinophils and other inflammatory cells also produce toxins that can directly damage and destroy LSCs.55
1.2.3.2. Atopic keratoconjunctivitis
Similarly to VKC, chronic ocular surface inflammation in atopic keratoconjunctivitis is associated with mechanical trauma and palpebral scarring that leads to LSCD.56 Neovascularization and lipid deposition typically affect the superior one-third of the cornea but can also become circumferential in severe cases.57 In addition, inflammatory cells produce toxins that can directly damage and destroy LSCs as described for VKC.
1.2.3.3. Graft-vs-host disease
Ocular complications are common in chronic graft-versus-host disease (GVHD) following allogeneic hematopoietic stem cell transplantation.58 Inflammation stimulates fibrosis in the limbal area, which is detrimental to LSCs, and associated dry eye further exacerbates the chronic inflammatory process with more damage. Chronic severe chronic GVHD could lead to LSCD.59
2. Hereditary Limbal Stem Cell Deficiency
A primary genetic defect is an uncommon etiology of LSCD (Table 2); however, it is important to be able to diagnose the LSCD early and manage it accordingly.
Table 2.
Staging of limbal stem cell deficiency based on clinical presentation.
| A | B | C | ||
|---|---|---|---|---|
| Stage I | Normal corneal epithelium within the central 5 mm zone of the cornea | <50% of limbal involvement | ≥50% but <100% limbal involvement | 100% of limbal involvement |
| Stage II | The central 5 mm zone of the cornea is affected | < 50% of limbal involvement | ≥50% but <100% limbal involvement | |
| Stage III | The entire corneal surface is affected |
2.1. Congenital Aniridia
Congenital aniridia occurs in 1 in 50,000 to 100,000 newborns around the world, and this condition is usually inherited as an autosomal dominant pattern or is due to sporadic mutations in the PAX6 gene. Patients with aniridia can have other associated eye problems, e.g. glaucoma, cataract, and foveal and optic nerve hypoplasia. The corneal surface and limbal stem cells function are normal at birth but LSCD or aniridia-associated keratopathy usually develops at teenage years and progress to total LSCD accompanied by stromal opacity over time. Autosomal dominant keratitis could be a variant of aniridia.60 This type of keratitis is characterized by a partial or complete absence of the iris, typically in both eyes, but a variant with minimally affected irides has been reported.61
2.2. Autoimmune polyendocrinopathy-candidiasis–ectodermal dystrophy/dysplasia
Autoimmune polyendocrinopathy-candidiasis–ectodermal dystrophy/dysplasia, also known as multiple endocrine deficiency and autoimmune polyendocrine syndrome type 1, is inherited in an autosomal recessive fashion, and the autoimmune component of the syndrome affects multiple organs including parathyroid, adrenal glands and ectodermal tissues. Keratitis secondary to LSCD can occur in a subpopulation of affected individuals.42,62,63
2.3. Other
Uncommon genetic disorders that can cause LSCD include xeroderma pigmentosum,64 dyskeratosis congenital,65 ectrodactyly-ectodermal dysplasia-clefting syndrome,66,67 keratitis–ichthyosis–deafness syndrome,68,69 lacrimo-auriculo-dento-digital syndrome (Levy-Hollister) syndrome,70 and epidermolysis bullosa,71,72 Other genetic disorders such as autosomal dominant keratitis and dominantly inherited keratitis which have been reported only once are not included in Table 2. Additional reports are needed to confirm the genetic etiology of LSCD in these diseases.
Diagnosis of limbal stem cell deficiency
1. Clinical Presentation
1.1. Symptoms of limbal stem cell deficiency
Patients with LSCD may be asymptomatic initially. When symptomatic, patients may describe any or all of the following: ocular discomfort, irritation, foreign body sensation, conjunctival redness, tearing, dryness, blepharospasm, pain, photophobia, decreased vision, and blindness.73 These symptoms can be debilitating and lead to a decreased HRQOL.
1.2. Signs of limbal stem cell deficiency
The absence of the corneal epithelium phenotype or conjunctivalization of the cornea produces clinical signs of LSCD. Slit lamp examination, particularly of the superior cornea in early disease, and fluorescein staining pattern could reveal signs of LSCD. Fluorescein staining of the ocular surface is a test that can differentiate between normal healthy corneal epithelium and abnormal pathologic epithelium. Under normal conditions, corneal epithelial cells on the surface are interconnected by tight junctions, which are impermeable to larger molecules. In contrast, the conjunctival epithelium is characterized by relatively loose cell-cell contacts, which result in a permeability that is up to 40 times greater than that of the corneal epithelium.74 In LSCD the epithelium on the corneal surface may be either conjunctival with neovascularization, a mixture of metaplastic corneal epithelial cells and conjunctival epithelial cells, or only conjunctival epithelial cells without neovascularization (Figure 1C, 1D).75,76 The conjunctival epithelium, which is thinner and hazy has a different protein profile that differs from those of the healthy cornea.77–79
The diagnosis of LSCD is aided significantly by the fluorescein staining pattern of the conjunctival epithelium. Under cobalt blue light, fluorescein allows to detect both the presence of abnormal epithelial cells in LSCD as well as their pattern of distribution. In contrast to the epithelial defects that are immediately stained by fluorescein, the dye diffuses into the paracellular space of the conjunctivalized surface and abnormal delayed staining is observed 10 or more minutes after fluorescein instillation.17,56,80 This abnormal staining pattern can be visualized even after rinsing with balanced salt solution or eye wash. In general, there is a marked difference in the thickness between the corneal epithelium, which tends to be regular and thick, and the conjunctival epithelium, which tends to be irregular and thin on the cornea (Figure 1D).79 This difference in thickness often sharply demarcates the area of conjunctivalization from the adjacent healthy corneal epithelium and is rendered more obvious by the pooling of fluorescein (Figure 1D).80
The pattern and intensity of fluorescein staining vary according to the severity of the disease. In mild or early disease, which often is sectoral, only punctate fluorescein staining in a curve-like path may be visible in the affected area (Figure 1D).32 As LSCD progresses and conjunctival-type epithelium spreads across the cornea, areas may have a dulled light reflex, an irregular and hazy opaque epithelium (easier seen with fluorescein staining under cobalt blue lighting), superficial vascularization of the cornea, and peripheral pannus (Figure 1E).76,81 In addition, absence or flattening of the limbal palisades of Vogt may be noted, although this finding can also be age-related.82 In the more severe stage, the abnormal epithelium spreads in a spiral pattern from the limbus and has been described as vortex keratopathy or whorl-like epitheliopathy.83 Characteristics of LSCD in its more advanced stages (Figure 1E, 1F) include subepithelial fibrosis, fibrovascular pannus, corneal stromal scarring and neovascularization (since conjunctival epithelium lacks anti-angiogenic properties compared to corneal epithelium), and persistent and/or recurrent epithelial defects.84,85
2. Diagnostic tests
There are limitations of slit lamp clinical examination and fluorescein staining test in the diagnosis of LSCD.86,87 The signs of abnormal epithelium can be subtle and often not specific to LSCD. Adjunct diagnostic tests are of great value in confirming LSCD whether or not associated with other ocular surface abnormalities such as tear deficiency, corneal neovascularization, keratinization, or if goblet cells are absent as determined by impression cytology and in vivo laser scanning confocal microscope (IVCM). Phenotypic analysis of cells sampled from the corneal surface by impression cytology or in vivo imaging can confirm the diagnosis of LSCD.
2.1. Cell sampling from the cornea to detect conjunctival cells
2.1.1. Cell sampling methods
Several methods can be used to obtain cells from the ocular surface. Impression cytology can collect cells only from the superficial layers of the epithelium.88,89 Cell scraping can collect cells from the deeper layers of the epithelium, which may reflect more accurately the phenotype of the epithelium under examination.90 Tissue biopsies can accurately provide the anatomic features of the ocular surface but is more invasive than other cell sampling methods; however, pathologic examination of surgically excised tissue may aid in definitely characterizing the pathophysiology of disease.
The use of impression cytology for the diagnosis of LSCD was established in 1989 by Tseng and coworkers.2,42 In the context of therapeutic strategies, impression cytology has been used to demonstrate preoperative conjunctivalization and postoperative restoration of the normal corneal phenotype after autologous limbal transplantation.19 Impression cytology is considered as the “gold standard” in diagnostic testing for LSCD. The method is simple, minimally invasive, and able to detect goblet cells or conjunctival epithelial cells on the corneal surface, which is the hallmark of LSCD. A filter paper made of nitrocellulose acetate, cellulose acetate, or hydrophilic Polytetrafluoroethylene (Biopore membrane) is placed on the cornea after instillation of topical anesthetics.
The sensitivity of impression cytology in the diagnosis of LSCD is affected by many factors: (I) The material used for sampling affects the outcome;91, 92 (II) The pressure of the application of the filter onto the ocular surface also affects the results of the sampling; (III) The location and size of sampling are important, especially in cases of sectoral LSCD; (V) Retrieval of conjunctival cells is more efficient than for corneal epithelial cells. Therefore, it is important to highlight that the absence of corneal epithelial cells on the filter paper does not necessarily indicate an absence of corneal epithelial cells on the ocular surface as is often the case in patients with normal corneas. IVCM, if available, could be used to detect residual limbal and corneal epithelial cells.86,93 Due to the above sampling bias, impression cytology is not a quantitative test to stage LSCD.
2.1.2. Evaluation of cell biomarkers
Conventional histochemistry
Traditionally, histologic stains such as hematoxylin and eosin (H&E) or periodic acid-Schiff stain (PAS) are used to detect goblet cells collected by impression cytology or cell scraping (Figure 2E, F). The presence of goblet cells is indicative of conjunctivalization of the cornea. However, conjunctival goblet cells may be completely absent in patients with other ocular conditions such as keratinization.94,95 Therefore, a positive corneal impression cytology result demonstrating the presence of goblet cells is a confirmatory of a diagnosis of LSCD, whereas a negative result cannot rule out LSCD. In these cases, additional tests to detect conjunctival epithelial cells are required for a definitive diagnosis of LSCD.
Figure 2.
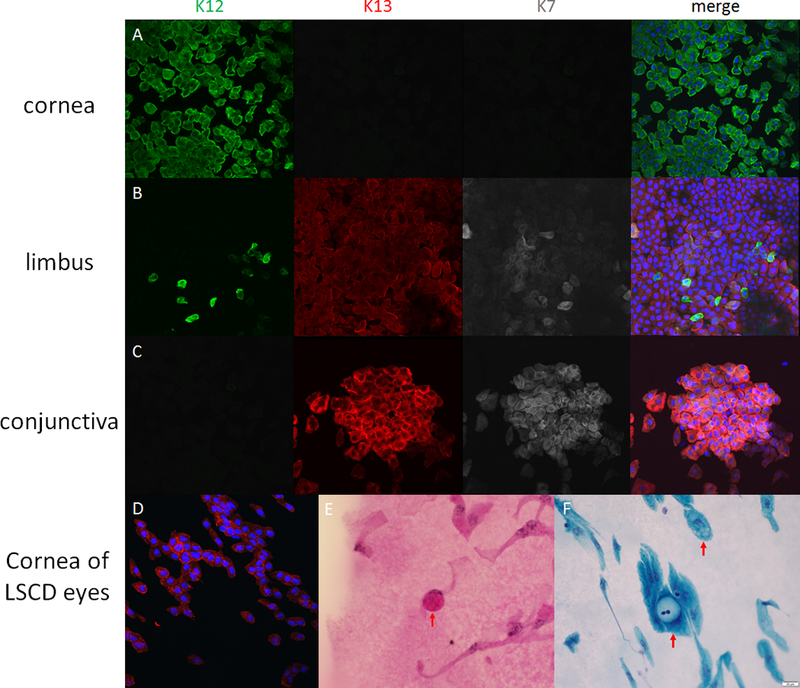
Immunofluorescein and pathological staining of corneal impression cytology specimens. Figure 2A, 2B and 2C are specimens taken from central cornea, limbus and conjunctiva of a normal cadeveric eye, respectively. The expression of cytokeratin (CK) 12 is only found in corneal epithelial cells, and cannot be found in conjunctival epithelial cells. CK13 and CK7 are only expressed in conjunctival cells. At the limbal area, both CK12-positive cells and CK13-CK7-positive cells are visible. Figure 2 D-F are the samples taken from the eyes with limbal stem cell deficiency. CK13 positive cells are visible on the cornea (2D). The goblet cells can be found through periodic acid-Schiff staining (2E) or Papanicolaou staining (2F) in the samples taken in limbal stem cell deficiency eyes after immunostaining. The goblet cells have 2–3 times larger cell size compared with epithelial cells, and mucus blobs are visible within the cells. The mucus blobs are usually visualized as pink by PAS staining.
Immunohistochemistry
Because of the low sensitivity of goblet cells in the diagnosis of LSCD, specific markers of conjunctival epithelial cells have been sought to indicate the presence of these cells on corneal impression cytology specimens. Immunohistochemistry allows detection of intracellular proteins (e.g., cytokeratins, CK) and secreted proteins that are typically expressed in conjunctival epithelial and goblet cells (e.g., mucins) 96. CK19 has been used in the past, but there are conflicting data regarding its specificity.97–101 Newer markers, (e.g., CK7,102 CK13,100,102,103 MUC1,97 and MUC5AC104) can also be used to support the diagnosis of LSCD (Figure 2 A–C).
Reverse transcription polymerase chain reaction
Reverse transcriptase polymerase chain reaction (RT-PCR) has been investigated as a method of detecting specific mRNA that are expressed in conjunctival cells collected by impression cytology. Because of its high sensitivity, RT-PCR can detect conjunctival markers such as CK19, CK7, CK13, MUC1, and MUC5AC. Using RT-PCR to detect MUC 5AC seems to be more sensitive than using immunocytology to detect goblet cells,104 if any.
2.2. In vivo Imaging techniques in the diagnosis of LSCD
2.2.1. In vivo confocal microscopy
In vivo laser scanning confocal microscopy has emerged as a diagnostic tool for LSCD, in part, because this method does not require the removal of corneal epithelial cells for analyses. IVCM provides information about disease severity and can be used in both the diagnosis and monitoring of LSCD. In addition, IVCM may be useful in evaluating the outcomes of cultured LSC grafts105 and recovery from trauma such as chemical injury.106
Corneal, conjunctival, and limbal epithelial cells can be distinguished on the basis of their different cell morphology.32,107 The absence of the corneal epithelium and/or the presence of conjunctival cells on the cornea is diagnostic of LSCD (Figure 3). Goblet cells can be detected by IVCM (Figure 3E).86 Because of the limitation in using goblet cells in the diagnosis of LSCD, the differences in cell morphology between the corneal and conjunctival epithelium detected by IVCM appears to be a more sensitive approach in diagnosing LSCD. Other features of IVCM findings suggestive of LSCD include epithelial thinning and reduction of subbasal nerve plexus and basal cell density.107–110
Figure 3.
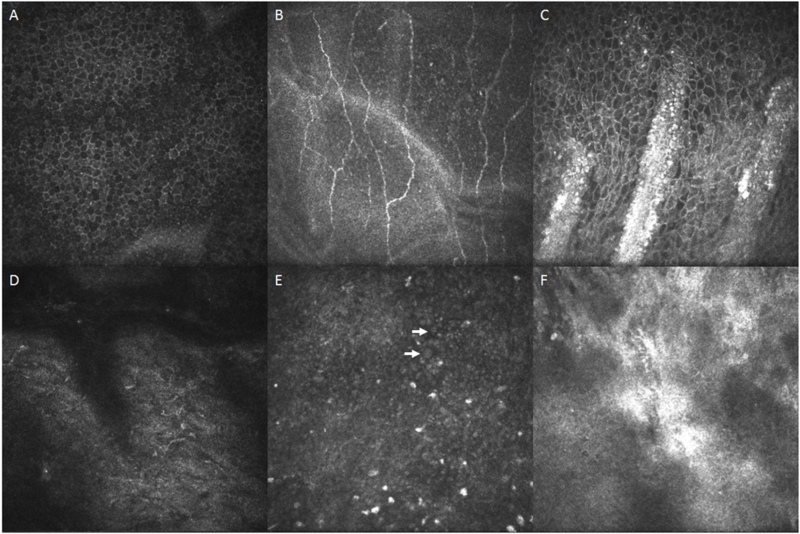
In vivo confocal microscopy imaging. Confocal microscopic images of normal eyes and eyes with limbal stem cell deficiency (LSCD). Panels A-C are images of normal eyes. Panels D-F are images of eyes with LSCD. Normal corneal basal epithelial cells (A) have well-defined, bright cell borders and hypo-reflective cytoplasm, with a regular and uniform arrangement. The nuclei are not visible or are very faint. Sub-basal nerve plexus (B) and the palisades of Vogt (C) are clearly visible in normal eyes. In eyes with severe LSCD, corneal basal epithelial cells are absence and sub-basal nerves were fragmented and the nerve density decreases significantly (D). Goblet cells (arrow) are occasionally visible (E). The palisades of Vogt (F) are not present in eyes with LSCD, but inflammatory cell infiltrates are sometimes visible.
2.2.2. Anterior segment optical coherence tomography
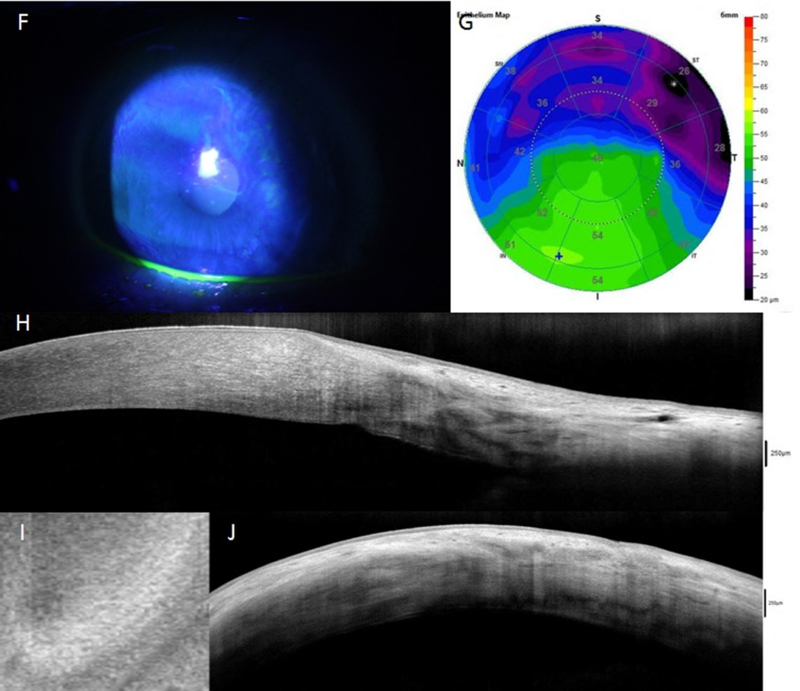
Anterior segment optical coherence tomography (AS-OCT) has emerged as an alternative imaging technique for LSCD, allowing for both noninvasive imaging of the ocular surface, including the limbus, and a larger field of view at the expense of resolution.111–115 Although AS-OCT does not offer the same degree of resolution at the cellular level as IVCM does, AS-OCT may prove useful in measuring epithelial thickness, pannus depth,116 assessing POV, limbal crypts, and the clear transition between the hyporeflective corneal epithelium and the hyperreflective conjunctival epithelium in the limbal region (i.e. cross-sections parallel and perpendicular to the limbus and en face sections).117,118 Compared with normal eyes, eyes with LSCD feature an absence of the POV and limbal crypts (limbal niche characteristics), and have greater variability in their central corneal epithelial thickness and, in general, a significant reduction in the thickness of limbal epithelium, as measured by AS-OCT (Figure 4).115,118 AS-OCT is also helpful in the diagnosis of LSCD with complex concomitant ocular surface diseases, such as ocular surface squamous neoplasia.119 Moreover, the results of AS-OCT are usually consistent with the findings of IVCM regarding the reduction of epithelial thickness or the morphologic changes in the POV.115,118 However, absence of POV is not pathonomognic for LSCD becasuse the presence of POV decreases with age even in normal eyes.82
Figure 4.
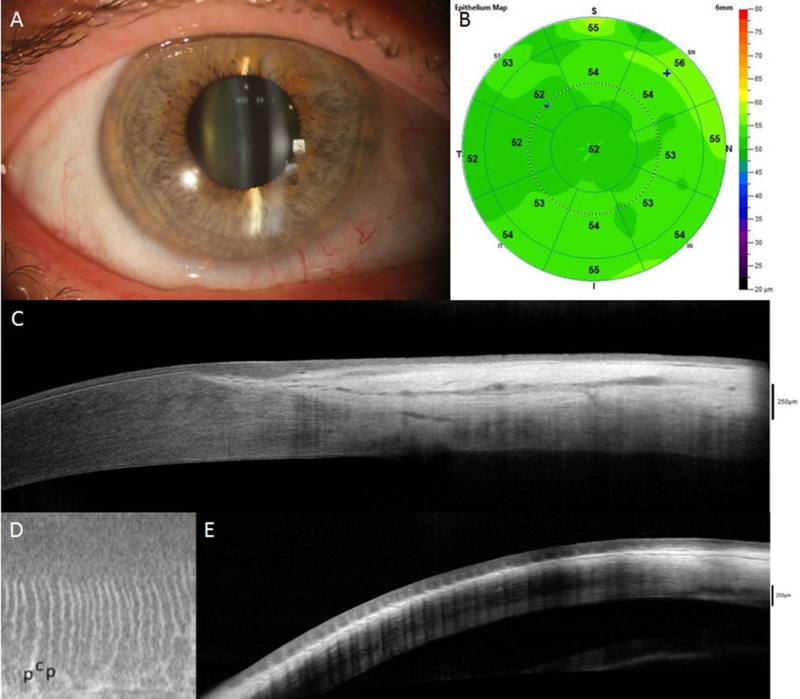
AS-OCT assessment of normal eyes (A-E) and eyes with limbal stem cell deficiency (LSCD, F-J). In eyes with sectoral LSCD, the corneal epithelial thickness in the affected area is decreased (G) compared to normal eye (B) and unaffected area (G); this reduction is consistent with the slit-lamp finding (F). Cross-sections perpendicular to the limbus show a clear transition between the hyporeflective corneal epithelium and the hyperreflective conjunctival epithelium with limbal epithelial thickening in normal eyes (C) but not in eyes with LSCD (H). The limbal epithelium in the affected area becomes thinner (H). The palisades of Vogt (p in figure) and limbal crypts (c in figure) are clearly visualized in normal eyes on en face mode (D) and parallel section (E), whereas they are absent in LSCD eyes (I and J).
2.2.3. Correlations between AS-OCT and IVCM, and impression cytology
In vivo confocal microscopy has a high degree of concordance with impression cytology in the diagnosis of LSCD.86 Because of cell sampling bias in impression cytology as metioned above, IVCM may prove to be more sensitive than impression cytology in detecting the presence of the corneal epithelium in sectoral or partial LSCD.86 This sensitivity is particularly important in staging LSCD accurately. In eyes with the clinical features of total LSCD as seen under slit-lamp biomicroscopy and confirmed by impression cytology, IVCM sometimes is able to detect well-demarcated, lacunae-like structures that are occasionally found in certain deep limbal stroma, which contain clusters of highly packed, normal limbal epithelial cells.93 However, the correlation between AS-OCT and impression cytology has not been investigated because the resolution of AS-OCT is not at the cellular level.
Staging of limbal stem cell deficiency based on clinical presentation
Limbal stem cell deficiency can be categorized into 3 stages based on the extent of corneal and limbal involvement detected by clinical examination as described in Table 2 and graphically illustrated in Figure 5. Staging of LSCD important to guide therapeutic recommendations and surgical planning. The most important factor is whether or not the visual axis or central 5 mm of cornea is affected (Stages II and III), and whether more than 50% of the limbal stem cells are intact should be considered. The final stage (Stage III) involves total LSCD where the whole corneal surface is affected. Abnormalities of other components of the ocular surface such as the conjunctiva, meibomian glands, lacrimal glands, tears, corneal nerves and immune system are important in the management of LSCD120 and will be addressed in a separate document on LSCD therapies.
Figure 5.
Staging system of limbal stem cell deficiency (LSCD). The severity of LSCD is classified into 3 stages. Stage I disease only involves the periphery of the cornea and the degree of involvement is staged into A, B and C (top panel). Stage II disease involves both the periphery and the central 5 mm of the cornea (second panel). Stage III disease involves the entire corneal surface (bottom panel).
Supplementary Material
Limbal Stem Cell Deficiency Etiologies. Indications of limbal stem cell transplantation in published literature are listed in the table.
Acknowledgements:
This work was supported by the Cornea Society.
Other members of LSCD working group who attended 2 or more meetings: Claus Cursiefen (University of Cologne, Cologne, Germany), Sheraz Daya (Centre for Sight, East Grinstead, United Kingdom), Ali Djalilian (University of Illinois at Chicago, IL, USA), Beatrice Frueh (University of Bern, Bern, Switzerland, Jesper Hjortdal (Aarhus University Hospital NBG, Aarhus, Denmark), Edward Holland (Cincinnati Eye Institute, University of Cincinnati, OH, USA), Stephen Kaufman (State University of New York-Downstate, NY, USA), Shigeru Kinoshita (Kyoto Prefectural University of Medicine, Kyoto, Japan), Barry Lee (Eye Consultants of Atlanta, GA, USA), Mark Mannis (University of California Davis, CA, USA), Jesus Merayo (Fernández-Vega Ophthalmological Institute, Oviedo, Spain), Victor Perez (Duke University School of Medicine, NC, USA), Paolo Rama (San Raffaele Scientific Institute, Milan, Italy), Virender Sangwan (LV Prasad Eye Institute, Hyderabad, India), Alex Shortt (University of College of London, The Royal Free Hospital, London, UK) Allan Slomovic (University of Toronto, Ontario, Canada), Avi Solomon (Hadassah-Hebrew University Medical Center, Jerusalem, Israel), Donald Tan (Singapore Eye Research Institute, Singapore), Ray Tsai (Taipei Eye Center and Taipei Medical University, Taipei, Taiwan), Scheffer Tseng (TissueTech Inc., Ocular Surface Center, and Ocular Surface Research and Education Foundation, Miami, Florida, USA), Elmer Tu (University of Illinois at Chicago, IL, USA)
Conflict of interest/disclosure:
CCC is a consultant or receives grants from Alcon Labs Inc, Allergan, Bausch & Lomb, Santen, Shire, Johnson & Johnson, Tearlab; VB is a consultant for Chiesi Farmaceutici S.P.A and Dompe; RD receives grant support from Allergan and is a consultant for Shire, Dompe, Santen; SXD receives grants from the National Eye Institute (R01EY021797) and California Institute for Regenerative Medicine (CLIN1–08686), and is a consultant for Chiesi Farmaceutici S.P.A; FCF is a consultant or receives research grants from Chiesi Farmaceutici S.P.A, Dompe, Santen, Shire and Théa; JAPG is a consultant or receives grant from Allergan, Alcon, MSD, Bausch & Lomb/Valeant, Mundipharma, Ofta Vision Health, Genon, Grin, Shire, FAPESP, Capes, Cnpq; PG is a shareholder of Holostem Terapie Avanzate and a consultant for J-TEC; SS receives research funds from Santen Pharmaceutical Co., Rohto Pharmaceuticals, Sumitomo Dainippon Pharma; FEK receives travel support from Santen Pharmaceutical Co., Ltd, and Kowa Company, LTD.
References
- 1.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tseng SC. Concept and application of limbal stem cells. Eye (Lond). 1989;3 ( Pt 2):141–157. [DOI] [PubMed] [Google Scholar]
- 3.Cotsarelis G, Cheng SZ, Dong G, et al. Existence of slow-cycling limbal epithelial basal cells that can be preferentially stimulated to proliferate: implications on epithelial stem cells. Cell. 1989;57:201–209. [DOI] [PubMed] [Google Scholar]
- 4.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001;98:3156–3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Paiva CS, Chen Z, Corrales RM, et al. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schofield R The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 7.Lane SW, Williams DA, Watt FM. Modulating the stem cell niche for tissue regeneration. Nat Biotechnol. 2014;32:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davanger M, Evensen A. Role of the pericorneal papillary structure in renewal of corneal epithelium. Nature. 1971;229:560–561. [DOI] [PubMed] [Google Scholar]
- 9.Schlotzer-Schrehardt U, Dietrich T, Saito K, et al. Characterization of extracellular matrix components in the limbal epithelial stem cell compartment. Exp Eye Res. 2007;85:845–860. [DOI] [PubMed] [Google Scholar]
- 10.Holan V, Pokorna K, Prochazkova J, et al. Immunoregulatory properties of mouse limbal stem cells. Journal of immunology (Baltimore, Md : 1950). 2010;184:2124–2129. [DOI] [PubMed] [Google Scholar]
- 11.Espana EM, Kawakita T, Romano A, et al. Stromal niche controls the plasticity of limbal and corneal epithelial differentiation in a rabbit model of recombined tissue. Invest Ophthalmol Vis Sci. 2003;44:5130–5135. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad S, Stewart R, Yung S, et al. Differentiation of human embryonic stem cells into corneal epithelial-like cells by in vitro replication of the corneal epithelial stem cell niche. Stem Cells. 2007;25:1145–1155. [DOI] [PubMed] [Google Scholar]
- 13.Polisetti N, Zenkel M, Menzel-Severing J, et al. Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem Cells. 2016;34:203–219. [DOI] [PubMed] [Google Scholar]
- 14.Maumenee AE, Scholz RO. The histopathology of the ocular lesions produced by the sulfur and nitrogen mustard. Bulletin of the Johns Hopkins Hospital. 1948;82:121–147. [PubMed] [Google Scholar]
- 15.Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:96–105. [PubMed] [Google Scholar]
- 16.Chen JJ, Tseng SC. Corneal epithelial wound healing in partial limbal deficiency. Invest Ophthalmol Vis Sci. 1990;31:1301–1314. [PubMed] [Google Scholar]
- 17.Tseng S, Chen J, Huang A, et al. Classification of conjunctival surgeries for corneal diseases based on stem cell concept. Ophthalmol Clin North Am. 1990;3:595–610. [Google Scholar]
- 18.Chen JJ, Tseng SC. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:2219–2233. [PubMed] [Google Scholar]
- 19.Kenyon KR, Tseng SC. Limbal autograft transplantation for ocular surface disorders. Ophthalmology. 1989;96:709–722; discussion 722–703. [DOI] [PubMed] [Google Scholar]
- 20.Shortt AJ, Bunce C, Levis HJ, et al. Three-year outcomes of cultured limbal epithelial allografts in aniridia and Stevens-Johnson syndrome evaluated using the Clinical Outcome Assessment in Surgical Trials assessment tool. Stem Cells Transl Med. 2014;3:265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. [DOI] [PubMed] [Google Scholar]
- 22.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. [DOI] [PubMed] [Google Scholar]
- 23.Daya SM, Chan CC, Holland EJ. Cornea Society nomenclature for ocular surface rehabilitative procedures. Cornea. 2011;30:1115–1119. [DOI] [PubMed] [Google Scholar]
- 24.Haring RS, Sheffield ID, Channa R, et al. Epidemiologic Trends of Chemical Ocular Burns in the United States. JAMA Ophthalmol. 2016;134:1119–1124. [DOI] [PubMed] [Google Scholar]
- 25.Fatima A, Iftekhar G, Sangwan VS, et al. Ocular surface changes in limbal stem cell deficiency caused by chemical injury: a histologic study of excised pannus from recipients of cultured corneal epithelium. Eye (Lond). 2008;22:1161–1167. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Garcia JS, Rivas Jara L, Garcia-Lozano I, et al. Histopathologic limbus evolution after alkaline burns. Cornea. 2007;26:1043–1048. [DOI] [PubMed] [Google Scholar]
- 27.Kadar T, Dachir S, Cohen L, et al. Ocular injuries following sulfur mustard exposure--pathological mechanism and potential therapy. Toxicology. 2009;263:59–69. [DOI] [PubMed] [Google Scholar]
- 28.Donisi PM, Rama P, Fasolo A, et al. Analysis of limbal stem cell deficiency by corneal impression cytology. Cornea. 2003;22:533–538. [DOI] [PubMed] [Google Scholar]
- 29.Sridhar MS, Vemuganti GK, Bansal AK, et al. Impression cytology-proven corneal stem cell deficiency in patients after surgeries involving the limbus. Cornea. 2001;20:145–148. [DOI] [PubMed] [Google Scholar]
- 30.Holland EJ, Schwartz GS. Iatrogenic limbal stem cell deficiency. Trans Am Ophthalmol Soc. 1997;95:95–107; discussion 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pires RT, Chokshi A, Tseng SC. Amniotic membrane transplantation or conjunctival limbal autograft for limbal stem cell deficiency induced by 5-fluorouracil in glaucoma surgeries. Cornea. 2000;19:284–287. [DOI] [PubMed] [Google Scholar]
- 32.Deng SX, Sejpal KD, Tang Q, et al. Characterization of limbal stem cell deficiency by in vivo laser scanning confocal microscopy: a microstructural approach. Arch Ophthalmol. 2012;130:440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lichtinger A, Pe’er J, Frucht-Pery J, et al. Limbal stem cell deficiency after topical mitomycin C therapy for primary acquired melanosis with atypia. Ophthalmology. 2010;117:431–437. [DOI] [PubMed] [Google Scholar]
- 34.Sauder G, Jonas JB. Limbal stem cell deficiency after subconjunctival mitomycin C injection for trabeculectomy. Am J Ophthalmol. 2006;141:1129–1130. [DOI] [PubMed] [Google Scholar]
- 35.Dudney BW, Malecha MA. Limbal stem cell deficiency following topical mitomycin C treatment of conjunctival-corneal intraepithelial neoplasia. Am J Ophthalmol. 2004;137:950–951. [DOI] [PubMed] [Google Scholar]
- 36.Lin Z, He H, Zhou T, et al. A mouse model of limbal stem cell deficiency induced by topical medication with the preservative benzalkonium chloride. Invest Ophthalmol Vis Sci. 2013;54:6314–6325. [DOI] [PubMed] [Google Scholar]
- 37.Ellies P, Anderson D, Topuhami A, et al. Limbal stem cell deficiency arising from systemic chemotherapy. Br J Ophthalmol. 2001;85:373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim K, Kim W. Corneal limbal stem cell deficiency associated with the anticancer drug S-1. Optom Vis Sci. 2015;92:S10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding X, Bishop R, Herzlich A, et al. Limbal stem cell deficiency arising from systemic chemotherapy with hydroxycarbamide. Cornea. 2009;28:221–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paris Fdos S, Goncalves ED, Barros Jde N, et al. Impression cytology findings in bullous keratopathy. Br J Ophthalmol. 2010;94:773–776. [DOI] [PubMed] [Google Scholar]
- 41.Uchino Y, Goto E, Takano Y, et al. Long-standing bullous keratopathy is associated with peripheral conjunctivalization and limbal deficiency. Ophthalmology. 2006;113:1098–1101. [DOI] [PubMed] [Google Scholar]
- 42.Puangsricharern V, Tseng SC. Cytologic evidence of corneal diseases with limbal stem cell deficiency. Ophthalmology. 1995;102:1476–1485. [DOI] [PubMed] [Google Scholar]
- 43.Dua HS, Azuara-Blanco A. Allo-limbal transplantation in patients with limbal stem cell deficiency. Br J Ophthalmol. 1999;83:414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lyall DA, Srinivasan S, Roberts F. Limbal stem cell failure secondary to advanced conjunctival squamous cell carcinoma: a clinicopathological case report. BMJ Case Rep. 2009;2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujishima H, Shimazaki J, Tsubota K. Temporary corneal stem cell dysfunction after radiation therapy. Br J Ophthalmol. 1996;80:911–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tseng SC, Tsubota K. Important concepts for treating ocular surface and tear disorders. Am J Ophthalmol. 1997;124:825–835. [DOI] [PubMed] [Google Scholar]
- 47.Ma KN, Thanos A, Chodosh J, et al. A Novel Technique for Amniotic Membrane Transplantation in Patients with Acute Stevens-Johnson Syndrome. Ocul Surf. 2016;14:31–36. [DOI] [PubMed] [Google Scholar]
- 48.Arafat SN, Suelves AM, Spurr-Michaud S, et al. Neutrophil collagenase, gelatinase, and myeloperoxidase in tears of patients with stevens-johnson syndrome and ocular cicatricial pemphigoid. Ophthalmology. 2014;121:79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Pascuale MA, Espana EM, Liu DT, et al. Correlation of corneal complications with eyelid cicatricial pathologies in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis syndrome. Ophthalmology. 2005;112:904–912. [DOI] [PubMed] [Google Scholar]
- 50.Mondino BJ, Brown SI. Ocular cicatricial pemphigoid. Ophthalmology. 1981;88:95–100. [DOI] [PubMed] [Google Scholar]
- 51.Eschle-Meniconi ME, Ahmad SR, Foster CS. Mucous membrane pemphigoid: an update. Curr Opin Ophthalmol. 2005;16:303–307. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt E, Meyer-Ter-Vehn T, Zillikens D, et al. [Mucous membrane pemphigoid with ocular involvement. Part I: Clinical manifestations, pathogenesis and diagnosis]. Ophthalmologe. 2008;105:285–297; quiz 298. [DOI] [PubMed] [Google Scholar]
- 53.Samson CM, Nduaguba C, Baltatzis S, et al. Limbal stem cell transplantation in chronic inflammatory eye disease. Ophthalmology. 2002;109:862–868. [DOI] [PubMed] [Google Scholar]
- 54.Sangwan V, Jain S, Vemuganti G, et al. Vernal keratoconjunctivitis with limbal stem cell deficiency. cornea. 2011;30:491–496. [DOI] [PubMed] [Google Scholar]
- 55.Sangwan VS, Basu S, Vemuganti GK, et al. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95:1525–1529. [DOI] [PubMed] [Google Scholar]
- 56.Solomon A, Ellies P, Anderson DF, et al. Long-term outcome of keratolimbal allograft with or without penetrating keratoplasty for total limbal stem cell deficiency. Ophthalmology. 2002;109:1159–1166. [DOI] [PubMed] [Google Scholar]
- 57.Daya SM, Ilari FA. Living related conjunctival limbal allograft for the treatment of stem cell deficiency. Ophthalmology. 2001;108:126–133; discussion 133–124. [DOI] [PubMed] [Google Scholar]
- 58.Dietrich-Ntoukas T, Cursiefen C, Westekemper H, et al. Diagnosis and treatment of ocular chronic graft-versus-host disease: report from the German-Austrian-Swiss Consensus Conference on Clinical Practice in chronic GVHD. Cornea. 2012;31:299–310. [DOI] [PubMed] [Google Scholar]
- 59.Meller D, Fuchsluger T, Pauklin M, et al. Ocular surface reconstruction in graft-versus-host disease with HLA-identical living-related allogeneic cultivated limbal epithelium after hematopoietic stem cell transplantation from the same donor. Cornea. 2009;28:233–236. [DOI] [PubMed] [Google Scholar]
- 60.Pearce WG, Mielke BW, Hassard DT, et al. Autosomal dominant keratitis: a possible aniridia variant. Can J Ophthalmol. 1995;30:131–137. [PubMed] [Google Scholar]
- 61.Skeens HM, Brooks BP, Holland EJ. Congenital aniridia variant: minimally abnormal irides with severe limbal stem cell deficiency. Ophthalmology. 2011;118:1260–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mohammadpour M, Javadi MA. Keratitis associated with multiple endocrine deficiency. Cornea. 2006;25:112–114. [DOI] [PubMed] [Google Scholar]
- 63.Merenmies L, Tarkkanen A. Chronic bilateral keratitis in autoimmune polyendocrinopathy-candidiadis-ectodermal dystrophy (APECED). A long-term follow-up and visual prognosis. Acta Ophthalmol Scand. 2000;78:532–535. [DOI] [PubMed] [Google Scholar]
- 64.Fernandes M, Sangwan VS, Vemuganti GK. Limbal stem cell deficiency and xeroderma pigmentosum: a case report. Eye (Lond). 2004;18:741–743. [DOI] [PubMed] [Google Scholar]
- 65.Aslan D, Ozdek S, Camurdan O, et al. Dyskeratosis congenita with corneal limbal insufficiency. Pediatr Blood Cancer. 2009;53:95–97. [DOI] [PubMed] [Google Scholar]
- 66.Tijmes NT, Zaal MJ, De Jong PT, et al. Two families with dyshidrotic ectodermal dysplasia associated with ingrowth of corneal vessels, limbal hair growth, and Bitot-like conjunctival anomalies. Ophthalmic Genet. 1997;18:185–192. [DOI] [PubMed] [Google Scholar]
- 67.Di Iorio E, Kaye SB, Ponzin D, et al. Limbal stem cell deficiency and ocular phenotype in ectrodactyly-ectodermal dysplasia-clefting syndrome caused by p63 mutations. Ophthalmology. 2012;119:74–83. [DOI] [PubMed] [Google Scholar]
- 68.Messmer EM, Kenyon KR, Rittinger O, et al. Ocular manifestations of keratitis-ichthyosis-deafness (KID) syndrome. Ophthalmology. 2005;112:e1–6. [DOI] [PubMed] [Google Scholar]
- 69.Gicquel JJ, Lami MC, Catier A, et al. [Limbal stem cell deficiency associated with KID syndrome, about a case]. J Fr Ophtalmol. 2002;25:1061–1064. [PubMed] [Google Scholar]
- 70.Cortes M, Lambiase A, Sacchetti M, et al. Limbal stem cell deficiency associated with LADD syndrome. Arch Ophthalmol. 2005;123:691–694. [DOI] [PubMed] [Google Scholar]
- 71.Pauklin M, Fuchsluger TA, Westekemper H, et al. Midterm results of cultivated autologous and allogeneic limbal epithelial transplantation in limbal stem cell deficiency. Dev Ophthalmol. 2010;45:57–70. [DOI] [PubMed] [Google Scholar]
- 72.Thanos M, Pauklin M, Steuhl KP, et al. Ocular surface reconstruction with cultivated limbal epithelium in a patient with unilateral stem cell deficiency caused by Epidermolysis bullosa dystrophica hallopeau-Siemens. Cornea. 2010;29:462–464. [DOI] [PubMed] [Google Scholar]
- 73.Holland EJ. Epithelial transplantation for the management of severe ocular surface disease. Trans Am Ophthalmol Soc. 1996;94:677–743. [PMC free article] [PubMed] [Google Scholar]
- 74.Huang AJ, Tseng SC, Kenyon KR. Paracellular permeability of corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1989;30:684–689. [PubMed] [Google Scholar]
- 75.Sacchetti M, Lambiase A, Cortes M, et al. Clinical and cytological findings in limbal stem cell deficiency. Graefes Arch Clin Exp Ophthalmol. 2005;243:870–876. [DOI] [PubMed] [Google Scholar]
- 76.Dua HS, Joseph A, Shanmuganathan VA, et al. Stem cell differentiation and the effects of deficiency. Eye (Lond). 2003;17:877–885. [DOI] [PubMed] [Google Scholar]
- 77.Harris TM, Berry ER, Pakurar AS, et al. Biochemical transformation of bulbar conjunctiva into corneal epithelium: an electrophoretic analysis. Exp Eye Res. 1985;41:597–605. [DOI] [PubMed] [Google Scholar]
- 78.Huang AJW, Tseng SCG, Farazdaghi M, et al. Modulation of paracellular permeability during corneal epithelial wound healing. ARVO Abstract. Invest Ophthalmol Vis Sci. 1986;27 (Suppl.) 54. [Google Scholar]
- 79.Dua HS. The conjunctiva in corneal epithelial wound healing. Br J Ophthalmol. 1998;82:1407–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dua HS, Gomes JA, Singh A. Corneal epithelial wound healing. Br J Ophthalmol. 1994;78:401–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim BY, Riaz KM, Bakhtiari P, et al. Medically reversible limbal stem cell disease: clinical features and management strategies. Ophthalmology. 2014;121:2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng T, Xu J. Age-related changes of human limbus on in vivo confocal microscopy. Cornea. 2008;27:782–786. [DOI] [PubMed] [Google Scholar]
- 83.D’Aversa G, Luchs JL, Fox MJ, et al. Advancing wave-like epitheliopathy. Clinical features and treatment. Ophthalmology. 1997;104:962–969. [DOI] [PubMed] [Google Scholar]
- 84.Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24:139–148. [DOI] [PubMed] [Google Scholar]
- 85.Ang AY, Chan CC, Biber JM, et al. Ocular surface stem cell transplantation rejection: incidence, characteristics, and outcomes. Cornea. 2013;32:229–236. [DOI] [PubMed] [Google Scholar]
- 86.Nubile M, Lanzini M, Miri A, et al. In vivo confocal microscopy in diagnosis of limbal stem cell deficiency. Am J Ophthalmol. 2013;155:220–232. [DOI] [PubMed] [Google Scholar]
- 87.Le Q, Samson CM, Deng SX. A Case of Corneal Neovascularization Misdiagnosed as Total Limbal Stem Cell Deficiency. Cornea. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Egbert PR, Lauber S, Maurice DM. A simple conjunctival biopsy. Am J Ophthalmol. 1977;84:798–801. [DOI] [PubMed] [Google Scholar]
- 89.Tseng SC. Staging of conjunctival squamous metaplasia by impression cytology. Ophthalmology. 1985;92:728–733. [DOI] [PubMed] [Google Scholar]
- 90.Tsubota K, Kajiwara K, Ugajin S, et al. Conjunctival brush cytology. Acta Cytol. 1990;34:233–235. [PubMed] [Google Scholar]
- 91.Vadrevu VL, Fullard RJ. Enhancements to the conjunctival impression cytology technique and examples of applications in a clinico-biochemical study of dry eye. The CLAO journal : official publication of the Contact Lens Association of Ophthalmologists, Inc. 1994;20:59–63. [PubMed] [Google Scholar]
- 92.Le Q, Xu J, Deng SX. The diagnosis of limbal stem cell deficiency. Ocul Surf. 2018;16:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan E, Le Q, Codriansky A, et al. Existence of Normal Limbal Epithelium in Eyes With Clinical Signs of Total Limbal Stem Cell Deficiency. Cornea. 2016;35:1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim KH, Park SW, Kim MK, et al. Effect of age and early intervention with a systemic steroid, intravenous immunoglobulin or amniotic membrane transplantation on the ocular outcomes of patients with Stevens-Johnson syndrome. Korean journal of ophthalmology : KJO. 2013;27:331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ohji M, Ohmi G, Kiritoshi A, et al. Goblet cell density in thermal and chemical injuries. Arch Ophthalmol. 1987;105:1686–1688. [DOI] [PubMed] [Google Scholar]
- 96.Espana EM, Di Pascuale MA, He H, et al. Characterization of corneal pannus removed from patients with total limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 2004;45:2961–2966. [DOI] [PubMed] [Google Scholar]
- 97.Barbaro V, Ferrari S, Fasolo A, et al. Evaluation of ocular surface disorders: a new diagnostic tool based on impression cytology and confocal laser scanning microscopy. Br J Ophthalmol. 2010;94:926–932. [DOI] [PubMed] [Google Scholar]
- 98.Chen Z, de Paiva CS, Luo L, et al. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kasper M, Moll R, Stosiek P, et al. Patterns of cytokeratin and vimentin expression in the human eye. Histochemistry. 1988;89:369–377. [DOI] [PubMed] [Google Scholar]
- 100.Ramirez-Miranda A, Nakatsu MN, Zarei-Ghanavati S, et al. Keratin 13 is a more specific marker of conjunctival epithelium than keratin 19. Mol Vis. 2011;17:1652–1661. [PMC free article] [PubMed] [Google Scholar]
- 101.Yoshida S, Shimmura S, Kawakita T, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47:4780–4786. [DOI] [PubMed] [Google Scholar]
- 102.Jirsova K, Dudakova L, Kalasova S, et al. The OV-TL 12/30 clone of anti-cytokeratin 7 antibody as a new marker of corneal conjunctivalization in patients with limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 2011;52:5892–5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poli M, Janin H, Justin V, et al. Keratin 13 immunostaining in corneal impression cytology for the diagnosis of limbal stem cell deficiency. Invest Ophthalmol Vis Sci. 2011;52:9411–9415. [DOI] [PubMed] [Google Scholar]
- 104.Garcia I, Etxebarria J, Boto-de-Los-Bueis A, et al. Comparative study of limbal stem cell deficiency diagnosis methods: detection of MUC5AC mRNA and goblet cells in corneal epithelium. Ophthalmology. 2012;119:923–929. [DOI] [PubMed] [Google Scholar]
- 105.Pedrotti E, Passilongo M, Fasolo A, et al. In Vivo Confocal Microscopy 1 Year after Autologous Cultured Limbal Stem Cell Grafts. Ophthalmology. 2015;122:1660–1668. [DOI] [PubMed] [Google Scholar]
- 106.Xiang J, Le Q, Li Y, et al. In vivo confocal microscopy of early corneal epithelial recovery in patients with chemical injury. Eye (Lond). 2015;29:1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Araujo AL, Ricardo JR, Sakai VN, et al. Impression cytology and in vivo confocal microscopy in corneas with total limbal stem cell deficiency. Arq Bras Oftalmol. 2013;76:305–308. [DOI] [PubMed] [Google Scholar]
- 108.Chan EH, Chen L, Yu F, et al. Epithelial Thinning in Limbal Stem Cell Deficiency. Am J Ophthalmol. 2015;160:669–677 e664. [DOI] [PubMed] [Google Scholar]
- 109.Chan EH, Chen L, Rao JY, et al. Limbal Basal Cell Density Decreases in Limbal Stem Cell Deficiency. Am J Ophthalmol. 2015;160:678–684 e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chuephanich P, Supiyaphun C, Aravena C, et al. Characterization of the Corneal Subbasal Nerve Plexus in Limbal Stem Cell Deficiency. Cornea. 2017;36:347–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bizheva K, Hutchings N, Sorbara L, et al. In vivo volumetric imaging of the human corneo-scleral limbus with spectral domain OCT. Biomed Opt Express. 2011;2:1794–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Espana EM, Djalilian AR, Yoo SH, et al. En face optical coherence tomography imaging of corneal limbal stem cell niche. - Clinical En Face OCT Atlas. 2013:77–79. [Google Scholar]
- 113.Haagdorens M, Behaegel J, Rozema J, et al. A method for quantifying limbal stem cell niches using OCT imaging. Br J Ophthalmol. 2017;101:1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bizheva K, Tan B, MacLellan B, et al. In-vivo imaging of the palisades of Vogt and the limbal crypts with sub-micrometer axial resolution optical coherence tomography. Biomed Opt Express. 2017;8:4141–4151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Le Q, Yang Y, Deng SX, et al. Correlation between the existence of the palisades of Vogt and limbal epithelial thickness in limbal stem cell deficiency. Clin Exp Ophthalmol. 2017;45:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zakaria N, Ni Dhubhghaill S, Taal M, et al. Optical Coherence Tomography in Cultivated Limbal Epithelial Stem Cell Transplantation Surgery. Asia Pac J Ophthalmol (Phila). 2015;4:339–345. [DOI] [PubMed] [Google Scholar]
- 117.Voskresenskaya A, Pozdeyeva N, Vasilyeva T, et al. Clinical and morphological manifestations of aniridia-associated keratopathy on anterior segment optical coherence tomography and in vivo confocal microscopy. Ocul Surf. 2017;15:759–769. [DOI] [PubMed] [Google Scholar]
- 118.Banayan N, Georgeon C, Grieve K, et al. Spectral Domain Optical Coherence Tomography in Limbal Stem Cell Deficiency. A Case Control Study. Am J Ophthalmol. 2018. [DOI] [PubMed] [Google Scholar]
- 119.Atallah M, Joag M, Galor A, et al. Role of high resolution optical coherence tomography in diagnosing ocular surface squamous neoplasia with coexisting ocular surface diseases. Ocul Surf. 2017;15:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holland EJ, Schwartz GS. The evolution of epithelial transplantation for severe ocular surface disease and a proposed classification system. Cornea. 1996;15:549–556. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Limbal Stem Cell Deficiency Etiologies. Indications of limbal stem cell transplantation in published literature are listed in the table.


