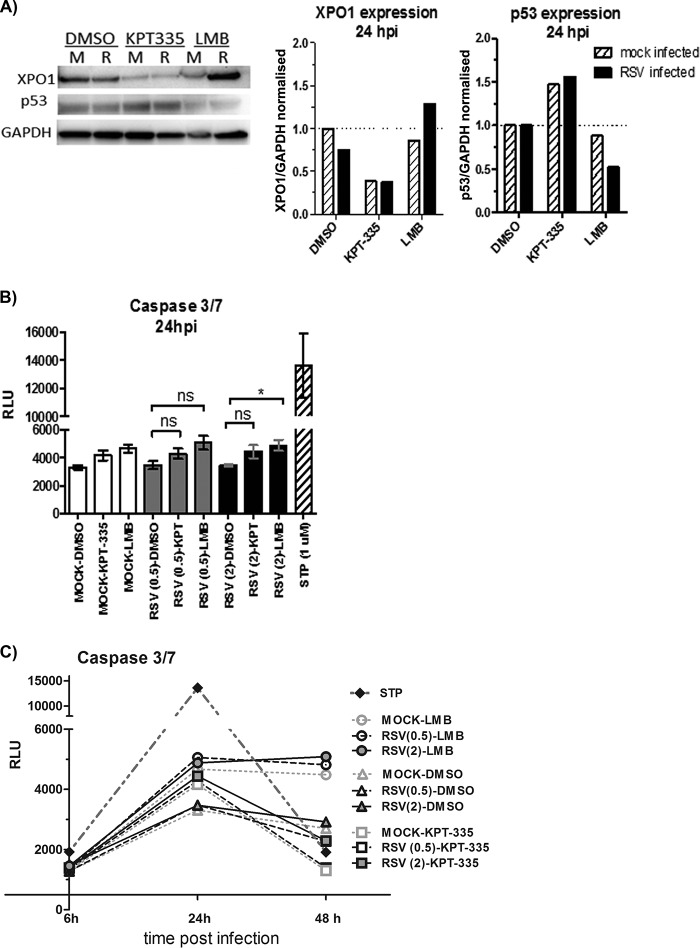
FIG 7.
KPT-335 reduces XPO1 expression and increases p53 expression without inducing apoptosis. (A) RSV-infected (R) or mock-infected (M) A549 cells were treated with 1 μM KPT-335, DMSO, or 750 nM LMB from 2 h p.i. Total cell lysate was collected at 24 h p.i. and analyzed by Western blotting as described in the legend of Fig. 5. Blots were probed for XPO1 and p53, with GAPDH used as a loading control. The primary antibodies used are indicated on the left. XPO1 and p53 expression was quantified using ImageJ, normalized to the level of GAPDH. Data shown are relative to values for DMSO-treated samples, taken as 1. (B) A549 cells were infected with RSV at an MOI of 0.5 or 2.0. KPT-335, DMSO, or LMB was added at 2 h p.i., and samples were collected at 24 h p.i. Assay controls included wells without cells (background), mock-infected cells (negative control), and cells treated with 1 μM staurosporine (STP) for 4 h (positive control). At 24 h p.i., plates were equilibrated at room temperature for 30 min, and 100 μl of Caspase-Glo 3/7 reagent was added to each well and incubated for 3 h at room temperature. Luminescence was measured using a Tecan microplate reader, and the background RLU count (wells without cells) was subtracted from each well. A Kruskal-Wallis one-way ANOVA was used to determine differences between groups, and statistically significant differences are indicated. *, P < 0.05; ns, nonsignificant (P > 0.05). (C) A549 cells were infected with RSV at an MOI of 0.5 or 2.0. KPT-335, DMSO, or LMB was added at 2 h p.i., and samples were collected at 6, 24, or 48 h p.i. At the indicated times plates were equilibrated at room temperature for 30 min, and 100 μl of Caspase-Glo 3/7 reagent was added to each well and incubated for 3 h at room temperature. Luminescence was measured using a Tecan microplate reader, and the background RLU count (wells without cells) was subtracted from each well.