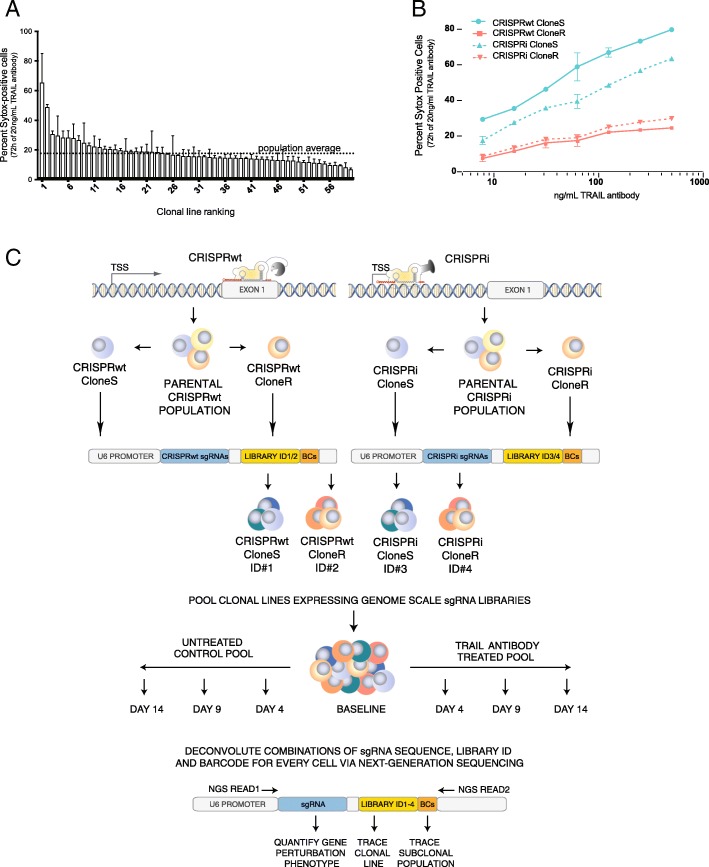
Fig. 1.
a Apoptosis response of clonal Cas9 Jurkat cell lines following 72 h treatment with 20 ng/μL TRAIL-R antibody, as assessed by Sytox staining and FACS. Standard deviation obtained from duplicate experiments is shown. Gating strategy for assessing Sytox is shown in Additional file 1: Figure S1. b Apoptosis response of the four clonal Cas9 lines used for pooled CRISPR screens at indicated concentrations of TRAIL-R antibody. Standard deviation is shown for duplicate experiments. c Schematic of clonal heterogeneity CRISPR screening and deconvolution approach. For CRISPRwt (top left) and CRISPRi (top right) the respective Cas9 gene was introduced into a population of Jurkat cells followed by the characterization of clonal lines for functionality of each CRISPR system. From both systems, two clonal lines were transduced with a multi-level barcoded sgRNA library (12 sgRNAs/gene) to knockout (CRISPRwt) or knockdown (CRISPRi) each protein coding gene in the human genome. Successfully transduced cells were pooled at equal numbers and the abundance of each clonal lines was traced via one of four library identifier sequences (ID) throughout the screen. Cell pools were cultured for 14 days in the absence (bottom left) or presence (bottom right) of TRAIL-R antibody. For downstream analysis via next-generation sequencing, sgRNA expression cassettes including the sgRNA encoding sequence, ID and BCs were recovered via PCR from the genomic DNA of cell pools from the beginning of the screen (baseline) as wells as on days 4, 9 and 14. Using a paired-end sequencing strategy allowed the quantification of the dis−/enrichment of sub-clonal populations (BC) within clonal lines (library ID) following the perturbation of any protein-coding gene in the human genome (sgRNA sequence)