Figure 2.
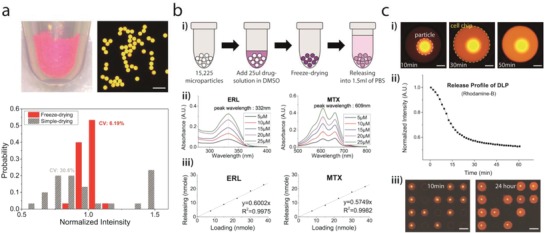
Drug delivery into the microwell using a microparticle. a) The loading uniformity of the freeze‐drying‐based loading method. Rhodamine‐B was used as a model substance, and the fluorescence intensity was measured to estimate the loading amount of molecules. The loading uniformity was significantly improved compared to a conventional drug loading method (see also Figure S2, Supporting Information). Scale bar: 500 µm. b) Measuring drug releasing ratio. (i) The releasing ratio of each drug was measured from a bulk‐scale releasing experiment. After drug loading by freeze‐drying, 1.5 mL of PBS was added to the DLPs. The concentration of released solution was measured after the overnight releasing process. (ii, iii) The UV–vis absorbance spectrum was used to measure the concentration of released solution. For all drugs, released amounts of drugs were linearly proportional to the initial loading amount (see also Figure S3 and Table S1, Supporting Information). Data of erlotinib and mitoxantrone are shown as representatives. c) Drug releasing into microwells and isolation of each microwell during incubation. (i) Image of a microwell with Rhodamine‐B releasing microparticle. Scale bar: 150 µm. (ii) Fluorescence intensity from a microparticle from time‐lapse imaging shows that the releasing process was completed within 30–60 min. (iii) The isolation of each microwell was maintained over the 24 h incubation time. Scale bar: 1 mm.
