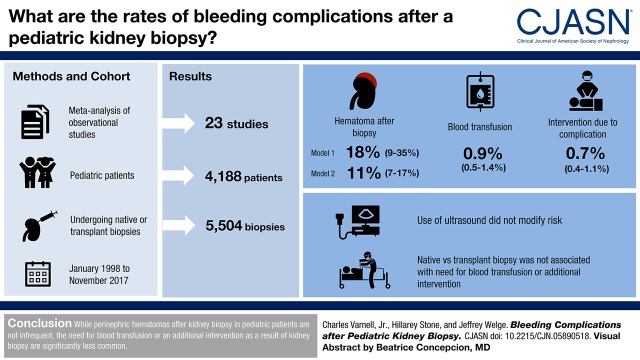
Abstract
Background and objectives
Kidney biopsy is an essential tool for the diagnosis and treatment of patients with kidney disease; however, because of its invasive nature, bleeding complications may arise.
Design, setting, participants, & measurements
We performed a meta-analysis of prospective or retrospective observational studies and randomized, controlled trials in pediatric patients undergoing native or transplant kidney biopsy in an inpatient or outpatient setting in MEDLINE-indexed studies from January 1998 to November 1, 2017 to determine the proportion of patients who develop hematoma, need blood transfusion, or need an additional intervention due to a complication after kidney biopsy.
Results
Twenty-three studies of 5504 biopsies met inclusion criteria. The proportion of patients developing hematoma after biopsy was between 11% (95% confidence interval, 7% to 17%) and 18% (95% confidence interval, 9% to 35%) using two analyses that included different time periods. The proportion needing blood transfusion was 0.9% (95% confidence interval, 0.5% to 1.4%). The proportion needing an additional intervention due to postbiopsy complication was 0.7% (95% confidence interval, 0.4% to 1.1%). Secondary analysis was not possible due to lack of data in the original manuscripts on laboratory values, needle gauges, number of needle passes, age of patient, or performer (attending versus trainee). Analysis with metaregression found that use of real-time ultrasound during biopsy did not modify the risk for hematoma, requirement of a blood products transfusion, or requirement of an additional procedure after biopsy. Analysis with metaregression comparing native biopsies with transplant biopsies did not reveal that biopsy type (native kidney biopsy versus transplant kidney biopsy) was associated with the need for a blood transfusion or requirement of an additional intervention after biopsy.
Conclusions
The development of perinephric hematoma after kidney biopsy is not an infrequent finding. The proportion of patients requiring blood transfusion or needing an additional intervention as a result of kidney biopsy in pediatric patients is significantly smaller.
Keywords: children, pediatric nephrology, kidney biopsy, renal biopsy, Retrospective Studies, Outpatients, Inpatients, MEDLINE, Prospective Studies, Kidney Diseases, Biopsy, kidney transplantation, Hematoma, Blood Transfusion
Visual Abstract
Introduction
Kidney biopsy is an essential tool for the diagnosis and treatment of patients with kidney disease. Conditions that may warrant biopsy include hematuria, proteinuria, nephrotic syndrome, acute nephritis, rapidly progressive GN, CKD of uncertain etiology, systemic diseases with kidney involvement (e.g., SLE), follow-up of treatment, and evaluation of transplanted kidneys for rejection and chronic injury (1). The use of spring-loaded biopsy needles is common due to their simplicity and ease of technique (1). The kidney is localized with ultrasound guidance, and the biopsy needle is advanced to the kidney capsule under ultrasound observation (1). The spring-loaded needle is activated, causing the obturator to automatically advance into the kidney, and the entire needle is removed with the tissue core (1). Two cores of kidney cortex are usually necessary for optimal evaluation, whereas three cores may be necessary if using smaller-gauge needles (1).
Despite its importance, kidney biopsy is an invasive procedure and can have unintended consequences. Complications of kidney biopsy include hematuria (both microscopic and macroscopic), perinephric hematoma formation, arteriovenous fistula, and inadvertent puncture of other viscera or major kidney vessels (1). Hematuria, perinephric hematoma, and small arteriovenous fistulae may or may not have clinical significance for the patient after a kidney biopsy.
When obtaining informed consent for a kidney biopsy, it is important to provide patients and families with accurate data on the likelihood of complications as well as the difference between what is and what is not clinically significant. Studies looking at complications of kidney biopsy in pediatrics are few and generally include small numbers of patients and procedures. The purposes of this study were to perform a systematic review of the literature for studies describing bleeding complications after kidney biopsy in pediatric patients in the era of real-time ultrasound guidance and to perform a meta-analysis of clinically relevant outcomes, with the ultimate goal to better inform patients and nephrologists about the likelihood of these complications.
Materials and Methods
Search Strategy
One author (C.D.V.) performed the literature search, completing a PubMed search of English language studies published over a 20-year period from January 1, 1998 to November 1, 2017. The medical subject heading terms and subheadings were ultrasonics, biopsy, child, kidney, ultrasonography, diagnostic imaging, and pathology. There were no search limits to allow for more studies to be manually reviewed to meet inclusion criteria. Additional studies were identified through a manual search of the bibliographies in retrieved studies. The search using PubMed started September 1, 2017 and was last accessed November 1, 2017.
Inclusion/Exclusion Criteria
The patient population was pediatric patients (as described by the individual manuscripts with no upper limit exclusion on the basis of age) who underwent percutaneous kidney biopsy (native or transplant) that used ultrasound for the procedure. Studies that described using “ultrasound guidance” were accepted in addition to “real-time ultrasound guidance.” Outcomes identified included gross hematuria, hematoma, arteriovenous fistula, need for blood transfusion, need for cystoscopy, need for embolization, need for surgery, or need for nephrectomy. Both retrospective and prospective study types were included. Editorials, review articles, and unpublished abstracts were not included.
Data Extraction
We extracted available data for complication rates and patient, procedural, and laboratory characteristics where available. Data were independently extracted by two authors (C.D.V. and H.K.S.) using premade data extraction forms in Microsoft Excel (2013), which were reviewed and confirmed by the two authors. Any discrepancies were discussed by the two data extractors, and they were resolved after mutual agreement was made.
Statistical Methods
Analysis was limited to studies that specifically included protocol postbiopsy ultrasound as part of their methods to identify the true incidence of hematoma after kidney biopsy and avoid bias of under-reporting (2–31). Hematoma was not necessarily clinically significant in each study analyzed. To evaluate for clinically significant complications postbiopsy, we evaluated for the percentage of patients in each study who required a blood transfusion or required an additional intervention related to a biopsy complication, including cystoscopy, embolization, surgery, or nephrectomy. For analysis, we used the DerSimonian and Laird random effects model using SAS PROC MIXED (version 9.4; SAS Institute Inc., Cary, NC). The scale for analysis was log odds to meet the assumptions of normality of the combined effect sizes, with results back transformed from that scale. Metaregression was performed for each outcome of interest to account for whether the outcomes were associated with the use of real-time ultrasound. When assessing for the proportion of patients needing blood transfusion or surgical intervention after biopsy, metaregression was performed to determine whether outcomes were associated with native kidney biopsies versus transplant kidney biopsies. Only one study (the study by Franke et al. [16]) in the hematoma outcomes analysis included a significant number of transplant kidney biopsies, and therefore, we did not perform metaregression to control for biopsy type related to this outcome. Forest plots were created using Microsoft Excel (2013) (2).
Missing/Incomplete Data
For metaregression, studies were assessed on whether they reported using real-time ultrasound during kidney biopsy. For studies where it was unclear (3–6), was not reported (7), or had a mixed population (8), the data were coded as if real-time ultrasound was not used. One study (9) reported that eight of 11 centers used real-time ultrasound; this study was treated as if real-time ultrasound was used.
Results
Study Flow
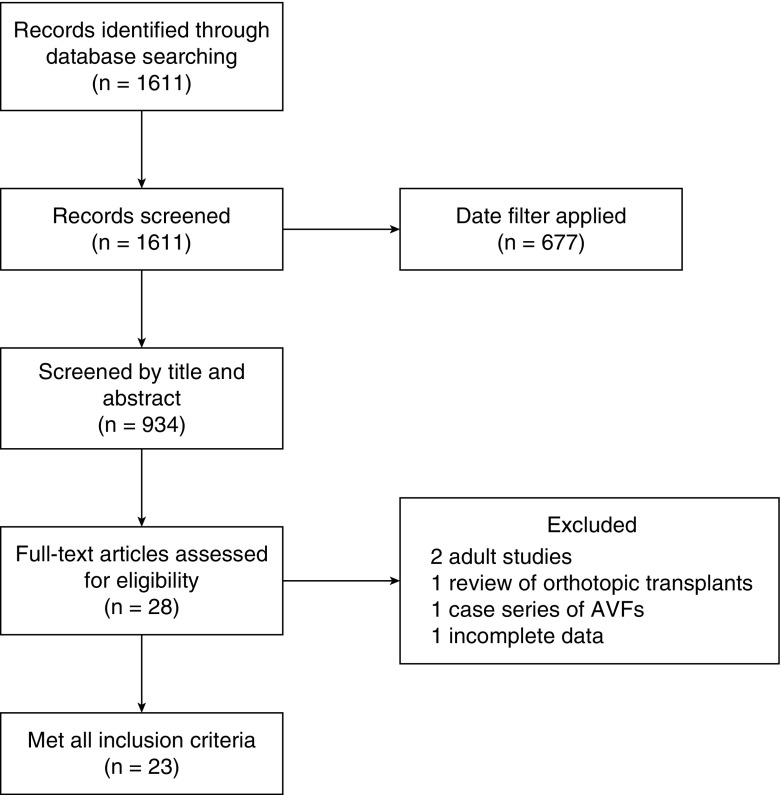
In total, 1611 studies were retrieved after initial search (Figure 1). After applying a date filter, there were 934 results that were screened by title and abstract. Of these, 906 studies were excluded, because their content was not relevant, which left 28 studies that were potentially relevant for full review. Of the 28 reviewed studies, two were excluded as adult studies (10,11). One was excluded, because it was a review of orthotopic transplant kidney biopsies (12). One was excluded, because it was a patient series describing arteriovenous fistulas without other demographic data (13), and one was excluded, because it did not report data in a complete and extractable manner (14). This left 23 studies that met full inclusion criteria. Baseline patient characteristics of included studies are detailed in Table 1, and selected procedure characteristics and complication rates are detailed in Table 2. The 23 included studies encompass 5504 biopsies in 4188 patients. Four of the studies were prospective, 18 were retrospective, one was unclear on retrospective versus prospective, and there were no randomized, controlled trials. Twenty of the 23 studies reported a mean or median age. Using the median age to represent the mean age in the three studies that did not report a mean age, the mean age of this cohort was 11 years old (SD of 1.8 years old), and the ages ranged from 15 days old to 24 years old. Sixteen of the 23 studies described the population by sex, with boys representing 51% of the cohort. Twenty-one of the 23 studies specified whether the biopsies were native or transplant kidney biopsies (n=5365), with 4237 (79%) native biopsies and 1128 (21%) transplant biopsies. Duration of monitoring for complications postbiopsy was specified in 19 of the 23 studies, with time ranging from 4 to 72 hours.
Figure 1.
Study flow diagram. AVF, arteriovenous fistula.
Table 1.
Baseline patient characteristics of included studies
| Author (Country) | Year | Study Design | No. of Procedures | No. of Native | No. of Transplant | Age, yra | Age Range | % Boys |
|---|---|---|---|---|---|---|---|---|
| Shah et al. (PK) (25) | 2015 | Unclear | 145 | 145 | 0 | 7.8 | 0.7–16 yr | 66 |
| Rianthavorn et al. (15) (TH) | 2014 | Prosp | 227 | 227 | 0 | 11b | NR | 42 |
| Franke et al. (16) (DE) | 2014 | Retro | 438 | 269 | 169 | 10.2 | 15 d to 23 yr | 56 |
| Yu et al. (17) (CN) | 2014 | Retro | 99 | 99 | 0 | 10.9 | NR | 53 |
| Gülcü et al. (18) (TR) | 2013 | Retro | 175 | 175 | 0 | 8.7 | 1–17 yr | 48 |
| Hirano et al. (19) (JP) | 2013 | Retro | 380 | 380 | 0 | 11 | NR | 55c |
| Tøndel et al. (3) (NO) | 2012 | Retro | 715 | 715 | 0 | 12 | 2 wk to 18 yr | NR |
| Printza et al. (20) (GR) | 2011 | Retro | 84 | 84 | 0 | 9.6 | 1–18 yr | 44 |
| Hussain et al. (9) (UK) | 2010 | Prosp | 531 | 352 | 179 | 11.8b | 1 mo to 18.9 yr | NR |
| Skalova and Rejtar (4) (CZ) | 2010 | Retro | 139d | 139 | 0 | 12.9 | 1–19 yr | 54 |
| Demircin et al. (21) (TR) | 2009 | Retro | 679 | 679 | 0 | 10.4 | 1 mo to 24 yr | 50 |
| Piotto et al. (22) (BR) | 2008 | Retro | 305 | 305 | 0 | 9.8 | 2–20 yr | 49 |
| Sweeney et al. (26) (CA) | 2006 | Retro | 65 | 0 | 65 | 14.5 | 3.2–18.6 yr | NR |
| Al Makdama et al. (30) (SA) | 2006 | Retro | 88 | 43 | 45 | NR | NR to 15 yr | 51 |
| Nammalwar et al. (5) (IN) | 2006 | Retro | 250 | 250 | 0 | <18 | NR | NR |
| Sinha et al. (28) (UK) | 2006 | Retro | 186e | 102 | 84 | 11.8b | 0.6–18.1 yr | 62 |
| Vidhun et al. (27) (US) | 2003 | Retro | 328 | 0 | 328 | 14.3 | 1–23 yr | NR |
| Sumboonnanonda et al. (31) (TH) | 2002 | Prosp | 90 | NR | NR | 7.8 | 16 mo to 16 yr | 55 |
| Kersnik Levart et al. (23) (SI) | 2001 | Retro | 87 | 81 | 6 | NR | 3–20 yr | 43 |
| Simckes et al. (7) (US) | 2000 | Prosp | 55 | 55 | 0 | 12.2 | NR | 58c |
| Benfield et al. (6) (US) | 1999 | Retro | 212 | 0 | 212 | 10.5 | 1.3–17.9 yr | NR |
| Riccabona et al. (24) (AT) | 1998 | Retro | 50 | NR | NR | 9.9 | 6 d–18.5 yr | 46 |
| Davis et al. (8) (US) | 1998 | Retro | 177 | 137 | 40 | 12.1 | NR | 47 |
PK, Pakistan; TH, Thailand; Prosp, prospective; NR, not reported; DE, Germany; Retro, retrospective; CN, China; TR, Turkey; JP, Japan; NO, Norway; GR, Greece; UK, United Kingdom; CZ, Czech Republic; BR, Brazil; CA, Canada; SA, Saudi Arabia; IN, India; US, United States; SI, Slovenia; AT, Austria.
Mean value unless otherwise noted.
Median value.
Denominator is number of biopsies due to how data were reported.
Study included 166 biopsies but only evaluated 139 for complications.
In total, 185 patients were assessed for complications.
Used data from time period “E,” which used ultrasound guidance.
Table 2.
Selected procedure characteristics and complication rates
| Author (Country) | Performer | Needle Gauge | No. of Needle Passes | Duration of Postbiopsy Monitoring | Gross Hematuria | Hematoma | Blood Transfusion | Arteriovenous Fistula | Cystoscopy/Embolization/Surgery | Loss of Kidney |
|---|---|---|---|---|---|---|---|---|---|---|
| Shah et al. (PK) (25) | PN, T | 16, 18a | NR | 8 h | 6 | 0 | 3 | NR | 0 | 0 |
| Rianthavorn et al. (15) (TH) | PN | 16 | 2–3 | 24 h | 46 | 58 | 2 | 0 | 1 | 0 |
| Franke et al. (16) (DE) | PN, T | 14, 16a | 1–3b | 24c; 4 hd | 5 | 6 | 3 | 7 | 6 | 0 |
| Yu et al. (17) (CN) | PN | 18 | 2 | >8 h | 3 | 11 | 0 | 0 | 0 | 0 |
| Gülcü et al. (18) (TR) | PN and R | 14 (97%); 16 (3%) | 1.8e | 24 h | 3 | 33 | 7 | 13 | 5 | 0 |
| Hirano et al. (19) (JP) | PN, T | 16 | NR | 72 h | 7 | 33 | NR | NR | NR | 0 |
| Tøndel et al. (3) (NO) | PR, R | 14 (18%); 16 of 18 (82%) | NR | NR | 11 | 58 | 1 | NR | 1 | 0 |
| Printza et al. (20) (GR) | NR | 16, 18a | 2 | 12 h | NR | 9 | 0 | NR | 0 | NR |
| Hussain et al. (9) (UK) | R, T | 16, 18a | NR | NR | 39 | NR | 4 | NR | 1 | 0 |
| Skalova and Rejtar (4) (CZ) | PN | NR | NR | 24 h | 5 | 30 | 0 | 0 | 0 | 0 |
| Demircin et al. (21) (TR) | NR | NR | NR | 24 h | 18 | 84 | 2 | 1 | 0 | 0 |
| Piotto et al. (22) (BR) | T | 16 (18%); 18 (82%) | 3.1e | 24 h | 17 | 26 | 0 | NR | 0 | 0 |
| Sweeney et al. (26) (CA) | PN and IR | 18 | 2 | 6–8 h | 8 | NR | 0 | 0 | 0 | 0 |
| Al Makdama et al. (30) (SA) | PN, T | 18 | NR | 4–6; 12 h | 3 | 1 | 3 | 3 | 1 | 0 |
| Nammalwar et al. (5) (IN) | NR | NR | NR | 24 h | 42 | 15 | 1f | NR | 1f | 1f |
| Sinha et al. (28) (UK) | PN, T | 14, 16, 18a | NR | 24 h | 13 | NR | 1 | NR | NR | 0 |
| Vidhun et al. (27) (US) | PN | 16; 18a | 2–3 | NR | 9 | 44 | 0g | NR | 0 | 0 |
| Sumboonnanonda et al. (31) (TH) | PN, T | 13 | 1–4 | 72 h | 21 | 1 | 2 | NR | 1 | NR |
| Kersnik Levart et al. (23) (SI) | PN | 18 (98%)h | 2.1e | 24 h | 1 | 55 | 0 | 0 | 0 | 0 |
| Simckes et al. (7) (US) | PN, T | 14, 15a | 4 | 4–6 versus 24 h | 0 | 20 | 0 | 0 | 0 | 0 |
| Benfield et al. (6) (US) | NR | NR | NR | NR | 6 | 1 | 2 | NR | 3 | 0 |
| Riccabona et al. (24) (AT) | NR | 14 | 2–3 | 48 h | NR | 46 | NR | 6 | 2 | 0 |
| Davis et al. (8) (US) | PN | 14, 15a | 1–2 | 23 h | 12 | 16 | 4 | NR | 0 | NR |
PK, Pakistan; PN, pediatric nephrologist; T, trainee; NR, not reported; TH, Thailand; DE, Germany; CN, China; TR, Turkey; R, radiologist; JP, Japan; PR, XXX; NO, Norway; GR, Greece; UK, United Kingdom; CZ, Czech Republic; BR, Brazil; CA, Canada; IR, interventional radiologist; SA, Saudi Arabia; IN, India; US, United States; SI, Slovenia; AT, Austria.
Percentages of each gauge not reported.
Most were from one to three; the range was from one to seven.
1997–2003.
2003–2011.
Average value reported.
Same patient.
Two patients were reported to have received blood transfusions postbiopsy as part of standard care of underlying disease (not due to biopsy).
The other 2% was not reported.
Development of Perinephric Hematoma Postbiopsy.
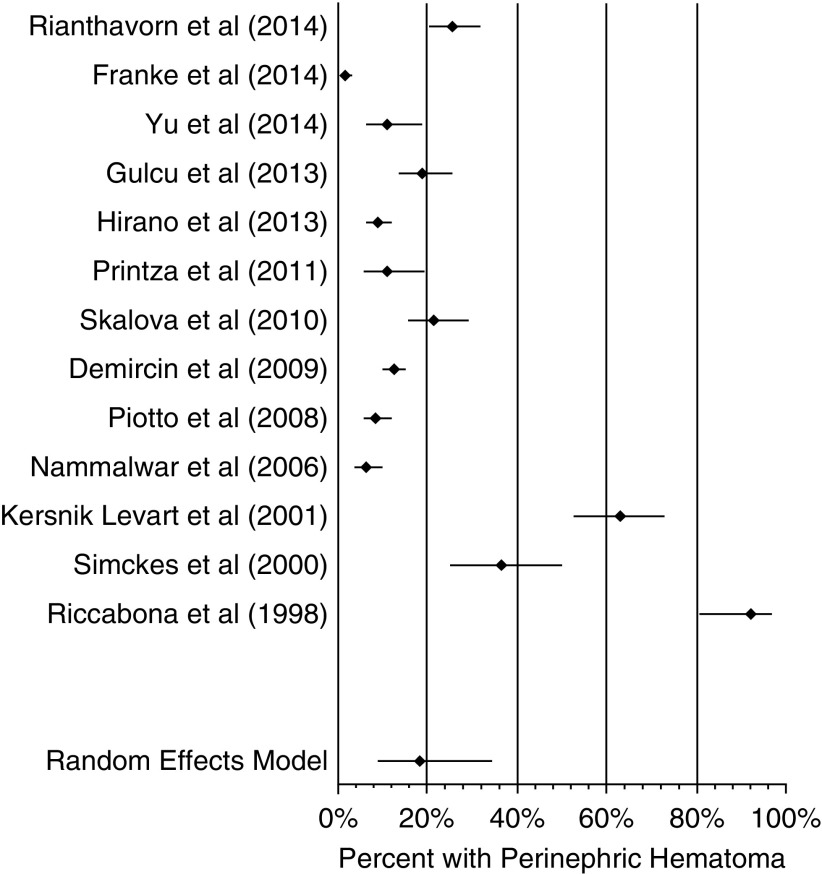
To assess for the development of perinephric hematoma after percutaneous kidney biopsy, studies that did not explicitly state that they evaluated the biopsied kidney with ultrasound after biopsy were excluded for this analysis. This left 13 studies (4,5,7,15–24) from our initial 23 included after systematic review, and these studies included 2968 biopsies. The proportion of patients who developed hematoma after biopsy was 18% (95% confidence interval [95% CI], 9% to 35%), Cochrane Q =289.9, I2=95.5%, and P<0.001 (Figure 2).
Figure 2.
Composite hematoma proportion was 18% (95% confidence interval, 9% to 35%). Forest plot of studies assessing for perinephric hematoma after ultrasound-assisted percutaneous kidney biopsy. Pooled hematoma proportion is displayed.
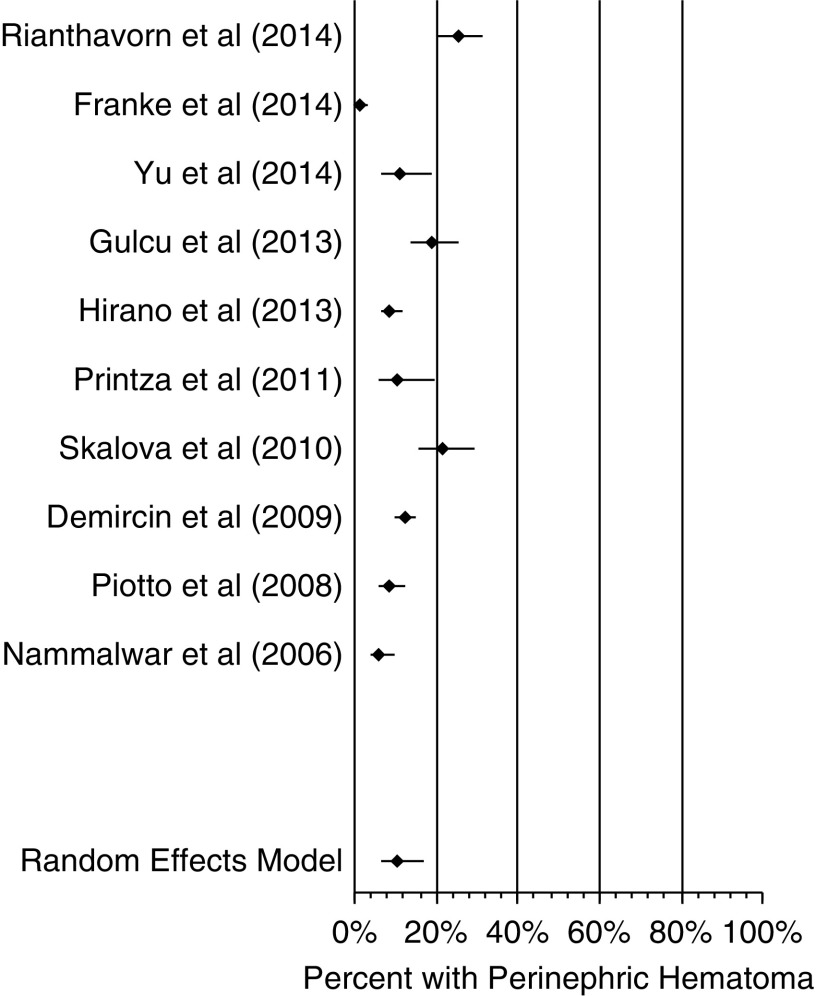
The forest plot in Figure 2 of perinephric hematoma after biopsy shows an obvious discrepancy between the studies from 1998 to 2001 and those from 2006 to 2014. The proportions of patients with hematoma were much higher in the studies by Riccabona et al. (24), Simckes et al. (7), and Kersnik Levart (23) at almost 40%–85% compared with the other studies in this analysis, which had around 20% or less. For sensitivity analysis, we assessed for the proportion of patients with hematoma after biopsy when excluding the three studies from 1998 to 2001 (Kersnik Levart et al. [23], Simckes et al. [7], and Riccabona et al. [24]). The model was repeated with the ten studies from 2006 to 2014, which included 2776 biopsies; the resultant forest plot is shown in Figure 3. In this second iteration, the proportion of patients with hematoma after biopsy was 11% (95% CI, 7% to 17%), Cochrane Q =135.7, I2=93.3%, and P<0.001.
Figure 3.
Composite perinephric hematoma proportion was 11% (95% confidence interval, 7% to 17%). Forest plot of studies assessing for perinephric hematoma after ultrasound-assisted percutaneous kidney biopsy. The studies by Kersnik Levart et al. (23), Simckes et al. (7), and Riccabona et al. (24) in Figure 2 seemed significantly different from the other studies, with much higher percentages of perinephric hematoma; they were removed, and analysis was repeated.
The proportion of patients with hematoma in the second model (11%) was almost one half the proportion of those with hematoma in the first model (18%). The average number of biopsies between the three studies from 1998 to 2001 was 64 compared with an average of 278 between the other studies analyzed. Simckes et al. (7) and Riccabona et al. (24) reported using larger 14-gauge needles, whereas only two (Franke et al. [16] and Gülcü et al. [18]) of the other ten studies in the hematoma analysis reported using 14-gauge biopsy needles. The remaining studies used either 16- or 18-gauge needles when needle size was reported (Table 2).
Need for Blood Transfusion Postbiopsy.
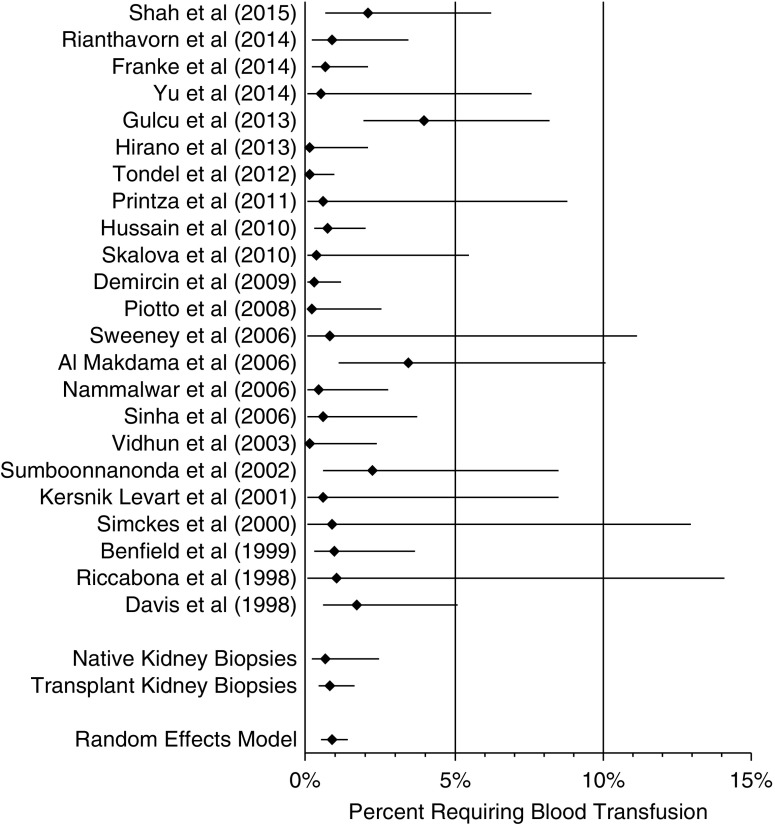
All 23 studies were included for analysis to evaluate the need for blood administration postbiopsy. One study by Shah et al. (25) included fresh frozen plasma administration in addition to blood administration; all other studies reported only blood transfusion. Given that multiple studies included a blood transfusion event of zero (4,7,17,19,20,22–24,26,27), a continuity correction of 0.5 was added to counts of zero to both event and nonevent rates for the purposes of this analysis. These 23 studies included 5504 total biopsies. The proportion of patients requiring blood transfusion after biopsy was 0.9% (95% CI, 0.5% to 1.4%), Cochrane Q =45.3, I2=49.3%, and P=0.002 (Figure 4). The risk for blood transfusion in native kidney biopsies was 0.6% (P=0.60; 95% CI, 0.2% to 2.4%), and the risk for blood transfusion in transplant kidney biopsies was 0.8% (P=0.70; 95% CI, 0.4% to 1.6%); these were not statistically different.
Figure 4.
Composite proportion was 0.9% (95% confidence interval, 0.5% to 1.4%). Forest plot of studies assessing for blood transfusion requirement after ultrasound-assisted percutaneous kidney biopsy. Pooled percentage requiring blood transfusion is displayed. In the subanalysis, the risk for blood transfusion in native kidney biopsies was 0.6% (95% confidence interval, 0.2% to 2.4%; P=0.60), and the risk for blood transfusion in transplant kidney biopsies was 0.8% (95% confidence interval, 0.4% to 1.6%; P=0.70); these were not statistically different from the composite proportion.
Complications Requiring an Additional Procedure or Intervention Postbiopsy.
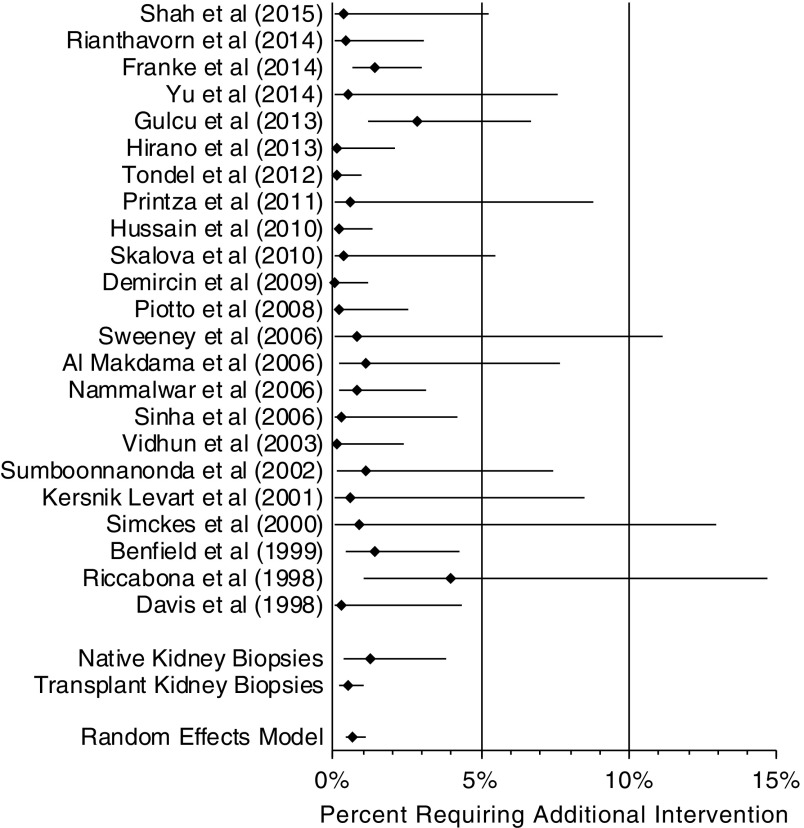
Need for an additional procedure or intervention was considered to be any report after biopsy of need for cystoscopy, need for embolization, need for surgery, or need for nephrectomy. All 23 studies were included for analysis. Given that multiple studies included an additional procedure event of zero (4,7,8,17,19–23,25–28) a continuity correction of 0.5 was added to counts of zero to both event and nonevent rates for the purposes of this analysis. If a value for need for an additional procedure or need for nephrectomy was not specifically reported, it was given a value of zero for the purposes of this analysis when “no serious side effects” was stated. These 23 studies included 5504 total biopsies. In the study by Nammalwar et al. (5), one patient developed subcapsular bleeding that required nephrectomy; the surgery and loss of kidney were only counted as one additional procedure, despite being listed separately in Table 1. The risk of additional intervention after biopsy was 0.7% (95% CI, 0.4% to 1.1%), Cochrane Q =32.1, I2=28.4%, and P=0.07 (Figure 5). The risk for additional intervention in native kidney biopsies was 1.2% (P=0.50; 95% CI, 0.4% to 3.8%), and the risk for additional intervention in transplant kidney biopsies was 0.5% (P=0.60; 95% CI, 0.2% to 1.1%); these were not statistically different.
Figure 5.
Composite proportion was 0.7% (95% confidence interval, 0.4% to 1.1%). Forest plot of studies assessing for additional intervention after ultrasound-assisted percutaneous kidney biopsy. Pooled percentage requiring additional intervention displayed. In the subanalysis, the risk for additional intervention in native kidney biopsies was 1.2% (95% confidence interval, 0.4% to 3.8%; P=0.50), and the risk for additional intervention in transplant kidney biopsies was 0.5% (95% confidence interval, 0.2% to 1.1%; P=0.60); these were not statistically different from the composite proportion.
Secondary Analyses
Secondary analysis was not possible on the basis of the available data for laboratory values, needle gauges, number of needle passes, age of patients, or performer (trainee versus attending physician), because these data either were not provided in the original studies or were aggregated and were not tied to outcomes. Metaregression was not performed to assess the difference in risk for the formation of hematoma after native versus transplanted kidney biopsies, because only one study in this analysis (Franke et al. [16]) included a significant number of transplanted kidneys. Use of real-time ultrasound during biopsy was not found to modify the risk for the development of hematoma after analysis with metaregression using the 13 studies listed in Figure 2. Real-time ultrasound use during biopsy did not modify the risk for blood transfusion (0.9%; 95% CI, 0.4% to 1.7%; P>0.99). Native (0.6%; 95% CI, 0.2% to 2.4%; P=0.60) versus transplant (0.8%; 95% CI, 0.4% to 1.6%; P=0.70) kidney biopsies did not modify the risk for blood transfusion. Real-time ultrasound use during biopsy did not modify the risk for an additional procedure (0.8%; 95% CI, 0.4% to 1.6%; P=0.50). Native (1.2%; 95% CI, 0.4% to 3.8%; P=0.50) versus transplant (0.5%; 95% CI, 0.2% to 1.1%; P=0.60) kidney biopsies did not modify the risk for an additional procedure.
Discussion
The results of this study present the proportion of patients developing hematoma formation after kidney biopsy as well as less common but clinically significant outcomes, such as need for blood transfusion or need for an additional procedure (e.g., cystoscopy, embolization, surgical exploration, or nephrectomy) as a result of a complication after kidney biopsy.
On the basis of our analyses, the percentage of patients who developed a hematoma after percutaneous ultrasound–assisted kidney biopsy is between 11% and 18%. The difference between these two analyses (Figure 2 versus Figure 3) is significant. There is an obvious visual discrepancy between the studies by Kersnik Levart et al. (23), Simckes et al. (7), and Riccabona et al. (24) with regards to their higher proportion of patients with a hematoma after biopsy. The average number of biopsies in these three studies was 64 compared with an average of 278 in the other studies analyzed, which may affect their rate. Another possible explanation of why these studies may have had a higher proportion of patients with a perinephric hematoma includes the use of larger 14-gauge needles in the studies by Simckes et al. (7) and Riccabona et al. (24), because these needle sizes have been reported in adults to have a higher risk of complications requiring blood transfusion compared with 16- or 18-gauge needles (29). Furthermore, these three studies were published in 1998, 2000, and 2001, making them the oldest in this meta-analysis. As technology (e.g., ultrasound resolution) improves over time and allows for better localization and real-time visualization, we would expect to see an improvement in complication rates.
It is important to note that, although hematoma is a bleeding complication of a kidney biopsy, it may or may not be clinically significant. Because it is so common in the literature, we included it in this analysis to estimate the true incidence of this complication in children. We then analyzed the need for a blood transfusion and the need for an additional procedure to describe clinically significant interventions needed as a consequence of kidney biopsy. It is reassuring to find that, although hematoma after kidney biopsy remains common (between 11% and 18%), clinically significant interventions, such as need for a blood transfusion (0.9%) or need for an additional procedure (0.7%), were much less likely. The meta-analysis performed by Corapi et al. (29) looked at bleeding complications of native kidney biopsy in 34 studies of 9474 biopsies in adults and reported that the rate of blood transfusion was 0.9% (95% CI, 0.4% to 1.5%), which is consistent with our finding in this pediatric meta-analysis.
Strengths of this study include the large total number of biopsies included for pooled analysis. One major limitation is the inability to control for potentially relevant sources of heterogeneity among the studies analyzed. This analysis would be made much more robust if we were able to control for biopsy needle characteristics (type, automated versus not, and gauge), performer (attending versus trainee and radiology versus nephrology), laboratory values, and patient age to assess outcomes in younger patients compared with older patients. These data largely either were not reported in individual studies or were reported in such a way that outcomes were not tied to multiple listed characteristics in the individual studies. An additional potential limitation that is included for all meta-analyses is publication bias, because centers with higher than commonly reported complication rates may be less likely to publish their results. Another limitation is that all reviewed studies were either retrospective or prospective observational studies; these types of studies may introduce a potential for bias compared with randomized, controlled trials, which may not be possible in this clinical situation.
At present, biopsy is the only means to establish a definitive diagnosis for many of the conditions for which pediatric nephrologists care. This study gives the most up to date outcomes regarding risk of hematoma as well as clinically significant outcomes, such as need for transfusion and need for additional intervention after ultrasound-assisted percutaneous kidney biopsy in pediatrics. These data are largely reassuring and will be helpful when discussing the biopsy procedure with patients and their families. Future studies that would enhance our understanding of complications of kidney biopsy in children would include more detailed prospective observational trials that specifically include data on the operator of the biopsy, type and size of biopsy needle, and the use of high-resolution ultrasound guidance and their effects on outcomes. It is reasonable to expect that, with more widespread use of improved ultrasound techniques for localization and automated biopsy needles, the safety of this often-essential technique in the practice of pediatric kidney disease management will continue to improve.
Disclosures
None.
Acknowledgments
This publication was supported by Institutional Clinical and Translational Science award 1UL1TR001425 from the National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS).
This work was completed in partial fulfillment of the Master of Science degree in Clinical and Translational Research in the Division of Epidemiology, University of Cincinnati College of Medicine.
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Avner ED, Harmon WE, Niaudet P, Yoshikawa N, Emma F, Goldstein SL: Pediatric Nephrology, 7th Ed., Berlin, Springer, 2016 [Google Scholar]
- 2.Clark O, Djulbegovic B: Forest Plots in Excel Software (Data Sheet), 2001. Available at www.evidencias.com. Accessed August 1, 2014
- 3.Tøndel C, Vikse BE, Bostad L, Svarstad E: Safety and complications of percutaneous kidney biopsies in 715 children and 8573 adults in Norway 1988-2010. Clin J Am Soc Nephrol 7: 1591–1597, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skalova S, Rejtar P: Safety profile of paediatric percutaneous ultrasonography-guided renal biopsies. Singapore Med J 51: 481–483, 2010 [PubMed] [Google Scholar]
- 5.Nammalwar BR, Vijayakumar M, Prahlad N: Experience of renal biopsy in children with nephrotic syndrome. Pediatr Nephrol 21: 286–288, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Benfield MR, Herrin J, Feld L, Rose S, Stablein D, Tejani A: Safety of kidney biopsy in pediatric transplantation: A report of the Controlled Clinical Trials in Pediatric Transplantation Trial of Induction Therapy Study Group. Transplantation 67: 544–547, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Simckes AM, Blowey DL, Gyves KM, Alon US: Success and safety of same-day kidney biopsy in children and adolescents. Pediatr Nephrol 14: 946–952, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Davis ID, Oehlenschlager W, O’Riordan MA, Avner ED: Pediatric renal biopsy: Should this procedure be performed in an outpatient setting? Pediatr Nephrol 12: 96–100, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Hussain F, Mallik M, Marks SD, Watson AR; British Association of Paediatric Nephrology : Renal biopsies in children: Current practice and audit of outcomes. Nephrol Dial Transplant 25: 485–489, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Ghnaimat M, Akash N, El-Lozi M: Kidney biopsy in Jordan: Complications and histopathological findings. Saudi J Kidney Dis Transpl 10: 152–156, 1999 [PubMed] [Google Scholar]
- 11.Rahbar M: Kidney biopsy in west of Iran: Complications and histopathological findings. Indian J Nephrol 19: 68–70, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mackie FE, Kennedy SE, Rosenberg AR, Kainer G, Frawley JE, Haghighi KS: Safety and adequacy of percutaneous biopsies in pediatric orthotopic kidney transplantation. Int J Organ Transplant Med 3: 26–31, 2012 [PMC free article] [PubMed] [Google Scholar]
- 13.Bilge I, Rozanes I, Acunas B, Minareci O, Nayir A, Oktem F, Tonguç E, Kozok Y, Emre S, Ander H, Sirin A, Poyanli A: Endovascular treatment of arteriovenous fistulas complicating percutaneous renal biopsy in three paediatric cases. Nephrol Dial Transplant 14: 2726–2730, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Feneberg R, Schaefer F, Zieger B, Waldherr R, Mehls O, Schärer K: Percutaneous renal biopsy in children: A 27-year experience. Nephron 79: 438–446, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Rianthavorn P, Kerr SJ, Chiengthong K: Safety of paediatric percutaneous native kidney biopsy and factors predicting bleeding complications. Nephrology (Carlton) 19: 143–148, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Franke M, Kramarczyk A, Taylan C, Maintz D, Hoppe B, Koerber F: Ultrasound-guided percutaneous renal biopsy in 295 children and adolescents: Role of ultrasound and analysis of complications. PLoS One 9: e114737, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu MC, Lee F, Huang WH, Hsueh S: Percutaneous ultrasound-guided renal biopsy in children: The need for renal biopsy in pediatric patients with persistent asymptomatic microscopic hematuria. Biomed J 37: 391–397, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Gülcü A, Göktay Y, Soylu A, Türkmen M, Kavukçu S, Seçil M, Karabay N: Doppler US evaluation of renal biopsy complications in children. Diagn Interv Radiol 19: 15–19, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Hirano D, Fujinaga S, Nishizaki N, Kanai H, Ida H: Role of ultrasound in revealing complications following percutaneous renal biopsy in children. Clin Nephrol 80: 426–432, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Printza N, Bosdou J, Pantzaki A, Badouraki M, Kollios K, Ghogha C, Papachristou F: Percutaneous ultrasound-guided renal biopsy in children: A single centre experience. Hippokratia 15: 258–261, 2011 [PMC free article] [PubMed] [Google Scholar]
- 21.Demircin G, Delibaş A, Bek K, Erdoğan O, Bülbül M, Baysun S, Oksal A, Memiş L, Oner A: A one-center experience with pediatric percutaneous renal biopsy and histopathology in Ankara, Turkey. Int Urol Nephrol 41: 933–939, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Piotto GHM, Moraes MCM, Malheiros DMAC, Saldanha LB, Koch VHK: Percutaneous ultrasound-guided renal biopsy in children - safety, efficacy, indications and renal pathology findings: 14-Year Brazilian university hospital experience. Clin Nephrol 69: 417–424, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Kersnik Levart T, Kenig A, Buturović Ponikvar J, Ferluga D, Avgustin Cavić M, Kenda RB: Real-time ultrasound-guided renal biopsy with a biopsy gun in children: Safety and efficacy. Acta Paediatr 90: 1394–1397, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Riccabona M, Schwinger W, Ring E: Arteriovenous fistula after renal biopsy in children. J Ultrasound Med 17: 505–508, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Shah SSH, Akhtar N, Ahmed T, Sunbleen F: Complications and safety of post percutaneous renal biopsy in paediatric patients. JAMC 27: 421–424, 2015 [PubMed] [Google Scholar]
- 26.Sweeney C, Geary DF, Hebert D, Robinson L, Langlois V: Outpatient pediatric renal transplant biopsy--is it safe? Pediatr Transplant 10: 159–161, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Vidhun J, Masciandro J, Varich L, Salvatierra O Jr., Sarwal M: Safety and risk stratification of percutaneous biopsies of adult-sized renal allografts in infant and older pediatric recipients. Transplantation 76: 552–557, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Sinha MD, Lewis MA, Bradbury MG, Webb NJA: Percutaneous real-time ultrasound-guided renal biopsy by automated biopsy gun in children: Safety and complications. J Nephrol 19: 41–44, 2006 [PubMed] [Google Scholar]
- 29.Corapi KM, Chen JL, Balk EM, Gordon CE: Bleeding complications of native kidney biopsy: A systematic review and meta-analysis. Am J Kidney Dis 60: 62–73, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Al Makdama A, Al-Akash S: Safety of Percutaneous Renal Biopsy as an Outpatient Procedure in Pediatric Patients. Annals of Saudi Medicine 26(4): 303–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sumboonnanonda A, Srajai K, Vongjirad A, Suntornpoch V, Parichatikanond P: Percutaneous Renal Biopsy in Children. J Med Assoc Thai 85(Suppl 2): S755–S761, 2002 [PubMed] [Google Scholar]