Abstract
Background and objectives
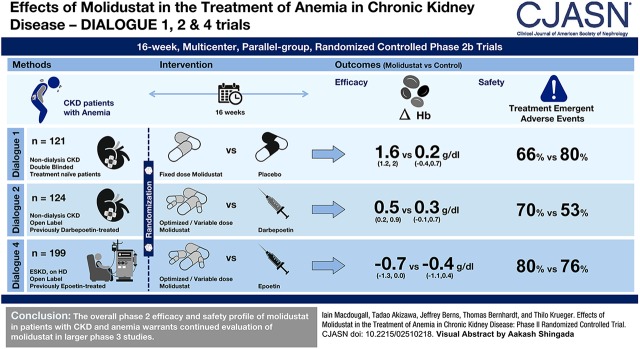
The efficacy and safety of molidustat, a hypoxia-inducible factor-prolyl hydroxylase inhibitor, have been evaluated in three 16-week, phase 2b studies in patients with CKD and anemia who are not on dialysis (DaIly orAL treatment increasing endOGenoUs Erythropoietin [DIALOGUE] 1 and 2) and in those who are on dialysis (DIALOGUE 4).
Design, setting, participants, & measurements
DIALOGUE 1 was a placebo-controlled, fixed-dose trial (25, 50, and 75 mg once daily; 25 and 50 mg twice daily). DIALOGUE 2 and 4 were open-label, variable-dose trials, in which treatment was switched from darbepoetin (DIAGLOGUE 2) or epoetin (DIALOGUE 4) to molidustat or continued with the original agents. Starting molidustat ranged between 25–75 and 25–150 mg daily in DIAGLOGUE 2 and 4, respectively, and could be titrated to maintain hemoglobin levels within predefined target ranges. The primary end point was the change in hemoglobin level between baseline and the mean value from the last 4 weeks of the treatment period.
Results
In DIAGLOGUE 1 (n=121), molidustat treatment was associated with estimated increases in mean hemoglobin levels of 1.4–2.0 g/dl. In DIAGLOGUE 2 (n=124), hemoglobin levels were maintained within the target range after switching to molidustat, with an estimated difference in mean change in hemoglobin levels between molidustat and darbepoetin treatments of up to 0.6 g/dl. In DIAGLOGUE 4 (n=199), hemoglobin levels were maintained within the target range after switching to molidustat 75 and 150 mg, with estimated differences in mean change between molidustat and epoetin treatment of −0.1 and 0.4 g/dl. Molidustat was generally well tolerated, and most adverse events were mild or moderate in severity.
Conclusions
The overall phase 2 efficacy and safety profile of molidustat in patients with CKD and anemia enables the progression of its development into phase 3.
Keywords: anemia; chronic kidney disease; hemoglobin; molidustat; Humans; Prolyl-Hydroxylase Inhibitors; Prolyl Hydroxylases; renal dialysis; Darbepoetin alfa; Epoetin Alfa; EPO protein; human; erythropoietin; Pyrazoles; Triazoles; Renal Insufficiency, Chronic; hypoxia
Visual Abstract
Introduction
Anemia is a common and important complication of CKD (1). As CKD worsens, the risk of anemia increases (2–4), owing to a combination of several conditions such as reduced erythrocyte lifespan and the reduced synthesis of erythropoietin (5,6). Erythropoiesis-stimulating agents (ESAs) mimic the actions of endogenous erythropoietin and are the standard treatment for patients with anemia of CKD (7). Although ESAs are effective in elevating hemoglobin levels, their use is associated with an increased risk of cardiovascular adverse events (AEs) (8–11). This appears to be related to the use of high doses to achieve specified hemoglobin targets (12,13), as well as excessive increases in hemoglobin levels (14). ESAs have also been shown to elevate BP in healthy volunteers and in patients with CKD (15). Furthermore, ESAs are ineffective at elevating hemoglobin levels in 10%–20% of patients, whether they are on dialysis (16,17) or not (18), largely owing to systemic inflammation and iron deficiency that inhibit the erythropoietic response (19).
Alternative treatment strategies are therefore worth pursuing for patients with anemia of CKD. One such approach is inhibition of hypoxia-inducible factor (HIF) prolyl hydroxylases (PHs). HIFs regulate the physiologic response to hypoxia by activating the transcription of erythropoietin and (to a lesser extent) several other hypoxia-inducible genes (20), which control multiple processes, including erythropoiesis, angiogenesis, and mitochondrial metabolism (19). In the presence of oxygen, HIF-PH tags HIF-α subunits for proteasomal degradation, and the transcription of target genes ceases (21,22).
Molidustat (BAY 85–3934) is a novel, orally bioavailable HIF-PH inhibitor that mimics hypoxia by stabilizing HIF-α subunits (20). Here, we report data from three phase 2b studies that are part of the DIALOGUE (DaIly orAL treatment increasing endOGenoUs Erythropoietin) program, which has been designed to evaluate the efficacy, safety, and tolerability of molidustat compared with placebo or alternative ESA therapy in patients with anemia of CKD.
Materials and Methods
DIALOGUEs 1, 2, and 4 were 16-week, multicenter, parallel group, randomized, controlled phase 2b trials in patients with anemia of CKD (Clinicaltrial.gov identifiers: NCT02021370, NCT02021409, and NCT01975818, respectively). An overview of each trial is provided in Table 1, with further details below and in Supplemental Tables 1–11.
Table 1.
Methodological overview of DIALOGUEs 1, 2, and 4
| Study Characteristics | DIALOGUE 1 | DIALOGUE 2 | DIALOGUE 4 | ||
|---|---|---|---|---|---|
| Clinicaltrials.gov identifier: NCT02021370a | Clinicaltrials.gov identifier: NCT02021409a | Clinicaltrials.gov identifier: NCT01975818b | |||
| Region | Europe and the Asia-Pacific | Europe and the Asia-Pacific | United States and Japan | ||
| Design | Randomized, double-blind, placebo-controlled, fixed-dose trial | Open-label, active comparator-controlled, variable-dose trial; comparator: darbepoetin alfa | Open-label, active comparator-controlled, variable-dose trial; comparator: epoetin alfa or beta | ||
| Patient population | Men and women (aged ≥18 yr) with a diagnosis of anemia of CKD | ||||
| Main inclusion criteria | ESA-naïve eGFRc <60 ml/min per 1.73 m2 but not on dialysis; mean Hb <10.5 g/dld | On stable dose of darbepoetin alfa eGFRc <60 ml/min per 1.73 m2 but not on dialysis; mean Hb 9.0–12.0 g/dle | On stable dose of epoetin alfa/beta; on dialysis; mean Hb 9.0–11.5 g/dlf | ||
| Treatment | Fixed doses of molidustat (25, 50, or 75 mg once daily; 25 or 50 mg twice daily) or placebo (1:1:1:1:1:1 randomization) | Molidustat (starting doses 25, 50, or 75 mg once daily) and plus optional 15, 100, and 150 mg or continued darbepoetin treatment (1:1:1:1 randomization) | Molidustat (starting doses 25, 50, 75, or 150 mgg and plus optional 15, 100, 200 mg once daily) or continued epoetin treatment (final allocation, 1:1:1:0.6:1) | ||
| Primary end point | Mean change in Hb level between baseline and the last 4 wk of treatment | ||||
| Key secondary end pointsh | Change in Hb during first 12 wk of treatment; rate of change of Hb over time | Response to treatmenti; time in target rangei; proportion of patients with Hb levels above and below target rangei | |||
DIALOGUE, DaIly orAL treatment increasing endOGenoUs Erythropoietin; ESA, erythropoiesis-stimulating agents; Hb, hemoglobin.
Registered December 2013.
Registered October 2013.
Defined according to the Modification of Diet in Renal Disease (31) or the formula devised by Matsuo et al. (32).
Mean of all local laboratory Hb measurements (at least two measurements must be taken ≥2 days apart) during the 4-week screening period, with the last screening Hb measurement within 10 days before randomization, all measurements come from the same local laboratory for any given subject, and the difference between the lowest value and the highest value is ≤1.2 g/dl.
Mean of all local laboratory Hb measurements (at least two measurements must be taken ≥2 days apart) during the 4-week screening period, none of the measurements can be <9.0 or >12.0 g/dl, none of the measurements can be <9.0 g/dl, all measurements must come from the same local laboratory for any given subject, and the difference between the lowest level and the highest level is ≤1.2 g/dl.
Mean of all local laboratory Hb measurements (at least two measurements must be taken ≥2 days apart) during the 4-week screening period, none of the measurements can be <9.0 or >12.0 g/dl, all measurements come from the same local laboratory for any given subject, and the difference between the lowest level and the highest level is ≤1.2 g/dl.
Added after a protocol amendment.
For a full list of secondary end points, see Supplemental Table 2.
Definitions of response and target range vary between DIALOGUE 2 and DIALOGUE 4; see Supplemental Table 2 for further information.
Study Design
The design of each study is summarized in Table 1. In DIALOGUE 1, which was placebo-controlled, the aim of treatment was to correct anemia. Both, patients and physicians were blinded to treatment allocation. DIALOGUE 2 and DIALOGUE 4 were open-label trials with the aim of maintaining hemoglobin levels within predefined ranges. The comparator was darbepoetin alfa (subsequently referred to as darbepoetin) in DIALOGUE 2 and epoetin alfa or beta (subsequently referred to as epoetin) in DIALOGUE 4.
All trials comprised a screening phase (≤4 weeks) and a randomized treatment phase (16 weeks, with an evaluation phase in the last 4 weeks) (Supplemental Figure 1). At the end of the treatment phase, patients were followed up for 8 weeks or (if eligible) could enter the DIALOGUE 3 or DIALOGUE 5 long-term extension studies. DIALOGUE 1 and DIALOGUE 2 were executed in the European Union, Israel, South Korea, Australia, and Japan, and DIALOGUE 4 enrolled patients in the United States and Japan.
Patient Populations
Key inclusion and exclusion criteria are summarized in Supplemental Table 1 and Table 1. All three trials enrolled adults with anemia of CKD. Patients in DIALOGUE 1 and DIALOGUE 2 were excluded if they were on dialysis, whereas DIALOGUE 4 only included patients undergoing long-term, regular hemodialysis. Participants in DIALOGUE 1 were not permitted to use ESAs in the 8 weeks before the start of the trial (considered sufficient time for pharmacological washout and loss of effects on erythropoiesis). In DIALOGUE 2 and DIALOGUE 4, patients had to be receiving stable doses of darbepoetin or epoetin, respectively, in the 8 weeks before randomization.
Treatments
In DIALOGUE 1, patients were treated with oral, fixed doses of molidustat or placebo. In DIALOGUE 2, patients were randomized to receive one of three starting doses once daily of oral molidustat (25, 50, or 75 mg) or to continue darbepoetin treatment. In DIALOGUE 4, patients were randomized to receive one of three starting doses of molidustat once daily (25, 50, or 75 mg) or to continue epoetin treatment. After initial efficacy results, a fourth arm was added (once daily 150 mg). Randomization was stratified according to previous thromboembolic events (all three studies) and hyporesponsiveness to darbepoetin or epoetin (DIALOGUE 2 and DIALOGUE 4, respectively).
Patients in DIALOGUE 1 discontinued the study if they experienced one of the following “stopping events”: hemoglobin level <8.0 g/dl, hemoglobin level at least 13.0 g/dl, or an increase in hemoglobin level of >1.0 g/dl in 2 weeks. In DIALOGUE 2 and DIALOGUE 4, the doses of all drugs could be titrated up or down in a stepwise manner, to maintain hemoglobin levels within predefined target ranges (Supplemental Figure 2). Darbepoetin and epoetin were titrated according to the local label. Except in emergencies, dose titration was only permitted on days 29, 57, and 85, although the dose could be reduced on day 15 if hemoglobin levels rose excessively. If hemoglobin became too high (>13.0 g/dl or a rise in hemoglobin of >1.0 g/dl in 2 weeks), molidustat could be titrated down or suspended. The daily average doses of molidustat in DIALOGUE 2 and DIALOGUE 4 are reported in Supplemental Table 2. In all studies, iron supplementation was per investigator discretion.
Efficacy End Points
The primary efficacy end point in each study was the change in hemoglobin level between baseline and the evaluation phase (the average of all measurements taken during the last 4 weeks of the treatment phase). Secondary efficacy end points are summarized in Supplemental Table 3 and Table 1.
Tolerability and Safety End Points
Safety and tolerability were assessed by evaluating AEs, heart rate, BP, electrocardiogram results, and laboratory parameters. The latter included measures of iron metabolism (iron, ferritin, total iron binding capacity, unsaturated iron binding capacity, transferrin saturation, and hepcidin) and plasma lipid levels. In addition, 24-hour ambulatory BP monitoring (ABPM) was conducted at baseline, at the end of week 8, and at the end of treatment in DIALOGUE 1 and in a subset of patients in DIALOGUE 4. An independent adjudication committee assessed all deaths and any serious AEs of severe arrhythmias, thromboembolic events, syncope or symptomatic hypotension, or heart failure.
Statistical Analyses
Efficacy was assessed using observed case data from the modified intention-to-treat set (all patients who were randomized, received at least one dose of study agent, and had at least one postbaseline efficacy value recorded). The estimates and the confidence intervals of the changes in hemoglobin levels were calculated using an analysis of covariance model. The safety analysis set (all patients who were randomized and received at least one dose of study agent) was used to analyze safety variables. Within each study, safety analyses were performed in individual and pooled molidustat dose groups. Sample sizes were on the basis of external benchmarks (e.g., sample sizes used in previous phase 2 studies of treatments for anemia of CKD) and feasibility, rather than statistical considerations. Further details of the statistical analyses are provided in Supplemental Material.
Ethics
All studies were conducted in compliance with the principles of the Declaration of Helsinki and in accordance with Good Clinical Practice guidelines. The protocols were reviewed and approved by the institutional review board or ethics committee of each participating center. All patients provided written informed consent before study entry.
Results
Patient Disposition and Baseline Characteristics
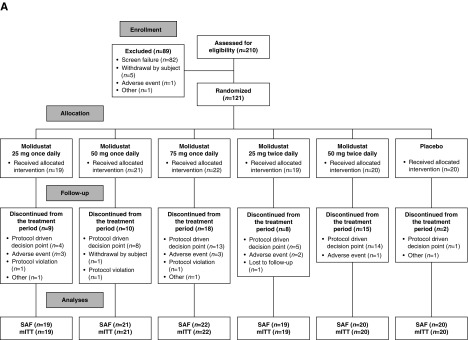
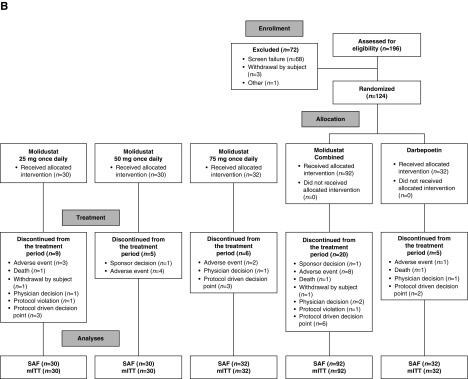
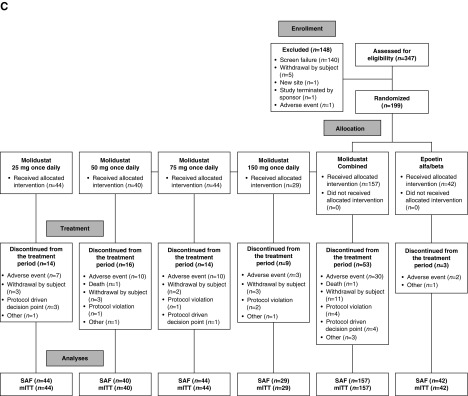
The numbers of patients randomized were 121 (DIALOGUE 1), 124 (DIALOGUE 2), and 199 (DIALOGUE 4). Patient disposition, including the number and reasons for discontinuation, during each of the three studies is shown in Figure 1. The numbers of patients in DIALOGUE 1 who discontinued because of stopping events (25 mg once daily, n=10; 50 mg once daily, n=9; 75 mg once daily, n=17; 25 mg twice daily, n=8; and 50 mg twice daily, n=17) were almost exclusively because of high hemoglobin levels.
Figure 1.
Patient disposition. mITT, modified intention-to-treat set; SAF, safety analysis set.
Baseline demographic and clinical characteristics are summarized in Supplemental Table 4 and Table 2. In all three studies, treatment groups were well balanced with respect to key baseline characteristics such as age, eGFR, and hemoglobin values.
Table 2.
Baseline demographics and clinical characteristics in DIALOGUEs 1, 2, and 4
| Patient Characteristics | DIALOGUE 1 | DIALOGUE 2 | DIALOGUE 4 | |||
|---|---|---|---|---|---|---|
| Molidustata (n=101) | Placebo (n=20) | Molidustata (n=92) | Darbepoetin (n=32) | Molidustata (n=157) | Epoetin (n=42) | |
| Mean age, yr (SD) | 69 (12) | 67 (16) | 68 (11) | 69 (9) | 59 (13) | 59 (9) |
| Women, n (%) | 45 (45) | 11 (55) | 47 (51) | 14 (44) | 66 (42) | 13 (31) |
| Race, n (%) | ||||||
| White | 63 (62) | 15 (75) | 69 (75) | 25 (78) | 84 (54) | 18 (43) |
| Asian | 38 (38) | 5 (25) | 22 (24) | 6 (19) | 29 (18) | 7 (17) |
| Black | 0 | 0 | 1 (1) | 1 (3) | 40 (25) | 17 (40) |
| Other | 0 | 0 | 0 | 0 | 4 (3) | 0 |
| Mean CKD duration, yr (SD) | 4.5 (4.5) | 3.5 (2.7) | 6.7 (6) | 5.8 (5) | 6 (6.2) | 6 (4.3) |
| Mean dialysis therapy duration, yr (SD) | – | – | – | – | 5 (5.2) | 5 (4.0) |
| CKD etiology, n (%)b | ||||||
| Diabetes | 45 (45) | 9 (45) | 31 (34) | 10 (31) | 86 (55) | 24 (57) |
| Hypertension | 45 (45) | 6 (30) | 31 (34) | 13 (41) | 48 (31) | 18 (43) |
| Mean eGFR, ml/min per 1.73 m2 (SD)c | 23 (12) | 23 (12) | 20 (11) | 22 (12) | ||
| Mean Hb level, g/dl (SD) | 9.5 (0.7) | 9.5 (0.6) | 10.8 (0.7) | 10.9 (0.7) | 10.5 (0.6) | 10.6 (0.5) |
| Mean prior ESA dose (µg/kg per wk) before randomization (SD) | – | – | 0.2 (0.2) | 0.3 (0.2) | 114 (98) | 103 (89) |
| Mean C-reactive protein, mg/L (SD) | 7.2 (16.4) | 4.3 (5.1) | 7.4 (14.9) | 6.5 (11.2) | 0.8 (1.4) | 0.7 (1.1) |
DIALOGUE, DaIly orAL treatment increasing endOGenoUs Erythropoietin; –, not applicable; Hb, hemoglobin; ESA, erythropoiesis-stimulating agents.
For DIALOGUE 2 and DIALOGUE 4, doses represent starting doses only.
Patients could have more than one etiology of CKD and the two most common etiologies are shown here. Other etiologies included autoimmune disease, cardiac diseases, GN, infection, and polycystic kidney disease.
eGFR was calculated using the Modification of Diet in Renal Disease formula.
Primary Efficacy End Point
The estimated mean changes and corresponding confidence intervals in hemoglobin level from baseline to the evaluation period for the three studies are summarized in Table 3.
Table 3.
Changes from baseline in mean local hemoglobin at evaluation period by starting dose in DIALOGUE 1, DIALOGUE 2, and DIALOGUE 4
| Within-Group Change from Baseline | Between-Group Comparison | ||||||
|---|---|---|---|---|---|---|---|
| Starting Dose Group | n | Baseline Mean | Evaluation Period Mean | LS Mean Change | 95% CI for LS Mean Change | Difference in LS Mean Change | 95% CI for Difference |
| DIALOGUE 1 | |||||||
| Molidustat 25 mg once daily | 12 | 9.5 | 10.9 | 1.4 | (0.7 to 2.1) | 1.3 | (0.5 to 2.0) |
| Molidustat 50 mg once daily | 11 | 9.7 | 11.1 | 1.5 | (0.7 to 2.3) | 1.3 | (0.5 to 2.0) |
| Molidustat 75 mg once daily | 5 | 10.0 | 11.8 | 1.8 | (0.9 to 2.7) | 1.7 | (0.7 to 2.7) |
| Molidustat 25 mg twice daily | 13 | 9.4 | 11.1 | 1.5 | (0.8 to 2.3) | 1.5 | (0.7 to 2.2) |
| Molidustat 50 mg twice daily | 9 | 9.4 | 11.2 | 2.0 | (1.1 to 2.8) | 1.7 | (0.8 to 2.5) |
| Molidustat combined | 50 | 9.5 | 11.1 | 1.6 | (1.2 to 2.0) | 1.4 | (0.9 to 2.0) |
| Placebo | 19 | 9.6 | 9.7 | 0.2 | (−0.4 to 0.7) | ||
| DIALOGUE 2 | |||||||
| Molidustat 25 mg once daily | 26 | 11.0 | 11.1 | 0.4 | (−0.1 to 1.0) | 0.0 | (−0.4 to 0.5) |
| Molidustat 50 mg once daily | 26 | 10.8 | 11.2 | 0.5 | (0.1 to 0.9) | 0.2 | (−0.2 to 0.6) |
| Molidustat 75 mg once daily | 28 | 10.7 | 11.5 | 0.9 | (0.5 to 1.3) | 0.6 | (0.2 to 0.9) |
| Molidustat combined | 80 | 10.8 | 11.3 | 0.5 | (0.2 to 0.9) | 0.2 | (−0.1 to 0.6) |
| Darbepoetin | 28 | 10.9 | 11.1 | 0.3 | (−0.1 to 0.7) | ||
| DIALOGUE 4 | |||||||
| Molidustat 25 mg once daily | 32 | 10.4 | 9.6 | −2.4 | (−3.4 to −1.4) | −0.8 | (−1.3 to −0.4) |
| Molidustat 50 mg once daily | 27 | 10.4 | 9.9 | −2.2 | (−3.2 to −1.2) | −0.5 | (−1.0 to −0.0) |
| Molidustat 75 mg once daily | 32 | 10.5 | 10.4 | −0.1 | (−1.0 to 0.7) | −0.1 | (−0.6 to 0.4) |
| Molidustat 150 mg once daily | 20 | 10.7 | 10.7 | −0.8 | (−1.4 to −0.1) | 0.4 | (−0.1 to 0.8) |
| Molidustat combined | 111 | 10.5 | 10.1 | −0.7 | (−1.3 to 0.0) | −0.3 | (−0.7 to 0.1) |
| Epoetin | 39 | 10.6 | 10.4 | −0.4 | (−1.1 to 0.4) | ||
n is number of subjects at evaluation period. LS mean and difference in LS mean are on the basis of analysis of covariance model including treatment, randomization stratification, factors, and baseline as a covariate. DIALOGUE, DaIly orAL treatment increasing endOGenoUs Erythropoietin; LS, least square; 95% CI, 95% confidence interval.
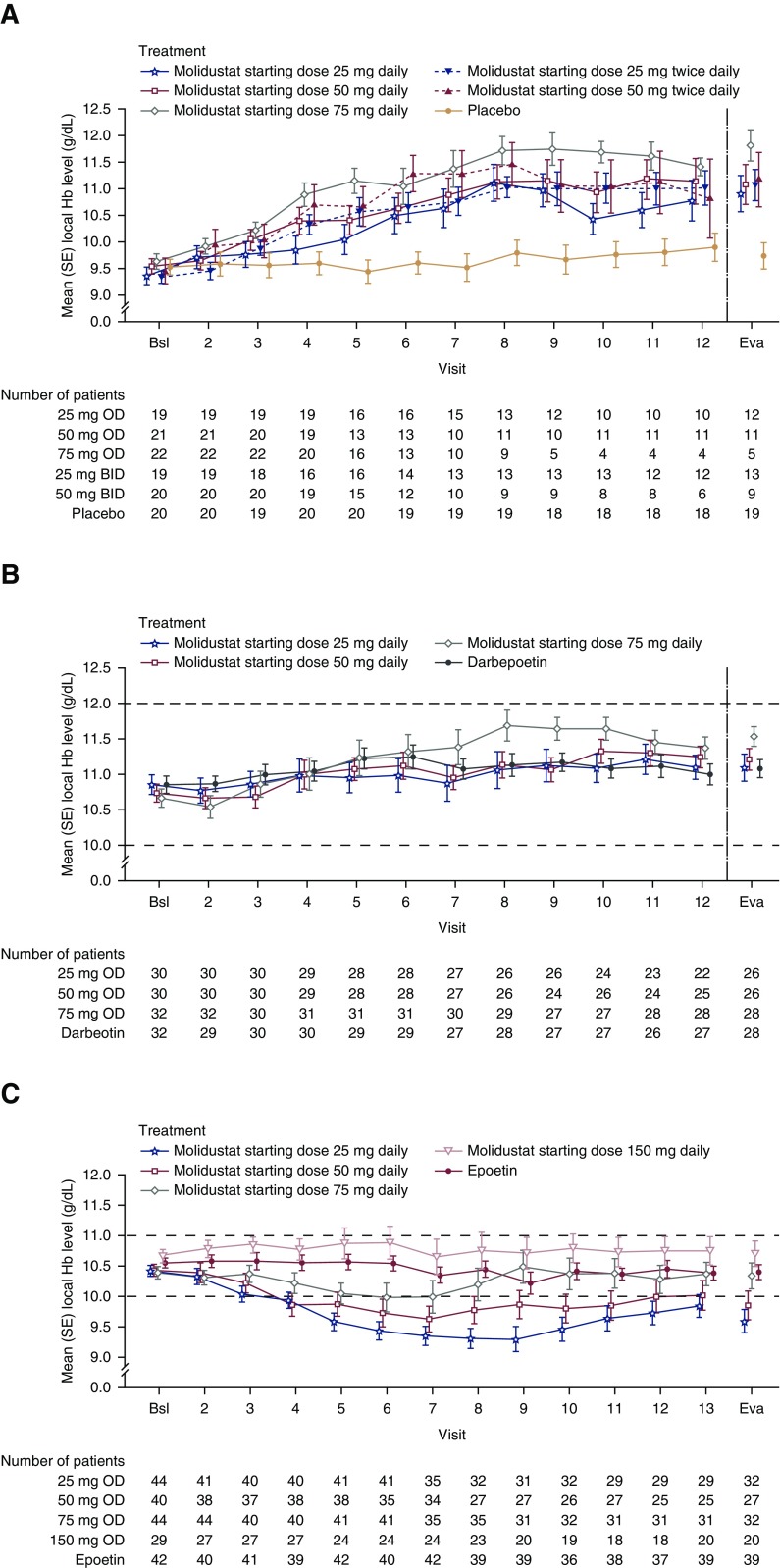
In DIALOGUE 1, mean hemoglobin levels in the molidustat groups increased from baseline during the first 12 weeks of treatment and began to separate from those of the placebo group at week 5. There were increases in hemoglobin level in each molidustat group between baseline and the evaluation period compared with placebo (Figure 2A, Table 3).
Figure 2.
Molidustat was able to correct (DIALOGUE 1) and maintain Hb levels within a pre-specified range (DIALOGUE 2 and 4 [75 and 150 mg starting dose groups only]). Observed case data from modified intention-to-treat populations. Baseline was defined as the average of all values before dosing with the first study agent. Dashed lines indicate target ranges. In DIALOGUE 2 and DIALOGUE 4, the doses of all drugs could be titrated up or down in a stepwise manner to maintain hemoglobin levels within predefined target ranges. Error bars indicate SEM. Bsl, baseline; Eva, evaluation period; Hb, hemoglobin.
In DIALOGUE 2, mean hemoglobin levels were maintained within the target range (10.0–12.0 g/dl) for each molidustat dose group (Figure 2B). The mean change in hemoglobin level between baseline and the evaluation period appeared to be numerically greater with the molidustat 75 mg once daily starting dose than with darbepoetin (Table 3).
In DIALOGUE 4, there was a numerically small mean increase in hemoglobin levels between baseline and the evaluation period in the molidustat 150 mg once daily group; there were small mean decreases in the other treatment groups (Table 3). Mean hemoglobin levels in the two lowest molidustat starting dose groups (25 and 50 mg once daily) fell below the target range (10.0–11.0 g/dl) in the first weeks of treatment, whereas mean hemoglobin levels for the 75 and 150 mg once daily starting doses remained within the target range during the 16-week treatment period (Figure 2C). Mean hemoglobin levels increased again toward the end of the treatment period in the 25 and 50 mg once daily groups, after dose titrations.
Secondary Efficacy End Points
In DIALOGUE 1, the increase in hemoglobin leveled off over time (Figure 2A); however, this apparent plateau has predominantly been influenced by high levels of protocol-defined patient discontinuations because of overshooting hemoglobin levels and/or exaggerated hemoglobin increase.
In DIALOGUE 2, the response rate (as defined in Supplemental Table 3) was numerically higher in the darbepoetin group (89%) than in the molidustat starting dose groups (71%, 81%, and 61% for the 25, 50, and 75 mg dose groups, respectively). The mean (SD) percentage of days within the hemoglobin target range (10–12 g/dl) were also numerically higher in the darbepoetin group (83%, SD 25) than in the molidustat dose groups (66%, SD 34; 71%, SD 31; and 56%, SD 30 for the 25, 50, and 75 mg dose groups, respectively), with no evidence of a dose-response effect with molidustat (Supplemental Table 6A). The proportion of patients with hemoglobin levels >12.0 g/dl was numerically higher with molidustat (15%, 12%, and 29% for the 25, 50, and 75 mg dose groups, respectively) than with darbepoetin (3.6%).
In DIALOGUE 4, the response rates (as defined in Supplemental Table 3) in the molidustat groups were variable and were numerically lower (43%, 22%, 19%, and 40.0% for the 25, 50, 75, and 150 mg dose groups, respectively) than that seen with epoetin (49%). The mean (SD) percentage of days within the hemoglobin target range of 10.0–11.0 g/dl were also numerically lower in the molidustat groups (34%, SD 29; 27%, SD 25; 27%, SD 26; and 38%, SD 28 for the 25, 50, 75, and 150 mg dose groups, respectively) than in the epoetin group (47%, SD 26), with no evidence of a dose-response effect with molidustat (Supplemental Table 6B). Compared with the epoetin group (23.1%), a numerically higher proportion of patients treated with the molidustat 150 mg once daily starting dose (40.0%) had hemoglobin levels >11.0 g/dl. Data on rescue treatment are given in Supplemental Table 7.
Tolerability and Safety
The mean duration of exposure to study agent was 75 days in the molidustat groups and 109 days in the placebo group in DIALOGUE 1, 105 days in the molidustat groups and 103 days in the darbepoetin group in DIALOGUE 2, and 95 days in the molidustat groups and 112 days in the epoetin group in DIALOGUE 4.
In DIALOGUE 1, the incidences of treatment-emergent adverse events (TEAEs) and serious TEAEs in the molidustat combined-dose group were numerically lower than those seen in the placebo group. The incidence of TEAEs in DIALOGUE 2 was numerically higher in the molidustat combined-dose group than in the darbepoetin group, and the incidence of study drug-related TEAEs in both DIALOGUE 2 and DIALOGUE 4 was numerically higher in the molidustat groups than in the darbepoetin/epoetin groups (Table 4). Most TEAEs in each of the three studies were mild or moderate in intensity. The most common TEAEs in each study are shown in Table 5 (common TEAEs by molidustat dose group are given in Supplemental Table 8).
Table 4.
Overall adverse event profile during treatment with molidustat or placebo/active comparator during DIALOGUEs 1, 2, and 4
| TEAEs | DIALOGUE 1 | DIALOGUE 2 | DIALOGUE 4 | |||
|---|---|---|---|---|---|---|
| Molidustata (n=101) | Placebo (n=20) | Molidustata (n=92) | Darbepoetin (n=32) | Molidustata (n=157) | Epoetin (n=42) | |
| Any TEAE | 67 (66) | 16 (80) | 64 (70) | 17 (53) | 126 (80) | 32 (76) |
| Any study drug-related TEAE | 12 (12) | 2 (10) | 8 (9) | 0 | 41 (26) | 4 (10) |
| Maximum intensity for any TEAE | ||||||
| Mild | 44 (44) | 7 (35) | 30 (33) | 7 (22) | 50 (32) | 12 (29) |
| Moderate | 18 (18) | 8 (40) | 29 (32) | 6 (19) | 43 (27) | 13 (31) |
| Severe | 5 (5) | 1 (5) | 5 (5) | 4 (13) | 33 (21) | 7 (17) |
| Any serious TEAE | 14 (14) | 5 (25) | 19 (21) | 6 (19) | 34 (22) | 7 (17) |
| Any study drug-related serious TEAE | 0 | 0 | 1 (1) | 0 | 2 (1) | 0 |
| Any TEAE resulting in death | 0 | 0 | 1 (1) | 1 (3) | 1 (1) | 0 |
| Any study drug-related TEAE resulting in death | 0 | 0 | 0 | 0 | 0 | 0 |
All data are given as n (%). All TEAEs reported were determined by the investigator. DIALOGUE, DaIly orAL treatment increasing endOGenoUs Erythropoietin; TEAE, treatment-emergent adverse event.
Combined-dose groups.
Table 5.
Treatment-emergent adverse events reported in >10% of patients in any group in DIALOGUEs 1, 2, and 4
| TEAEs | Molidustat Combined-Dose Group | Control Groupa |
|---|---|---|
| DIALOGUE 1 | n=101 | n=20 |
| Hyperparathyroidism, secondary | 1 (1) | 3 (15) |
| Constipation | 5 (5) | 1 (5) |
| Diarrhea | 4 (4) | 1 (5) |
| Vomiting | 2 (2) | 1 (5) |
| Nasopharyngitis | 7 (7) | 2 (10) |
| Urinary tract infection | 4 (4) | 3 (15) |
| Hyperkalemia | 4 (4) | 3 (15) |
| Dizziness | 5 (5) | 3 (15) |
| Hypertension | 10 (10) | 5 (25) |
| DIALOGUE 2 | n=92 | n=32 |
| Diarrhea | 5 (5) | 1 (3) |
| Edema, peripheral | 8 (9) | 2 (6) |
| CKD | 10 (11) | 0 |
| Hypertension | 14 (15) | 4 (13) |
| DIALOGUE 4 | n=157 | n=42 |
| Diarrhea | 12 (8) | 2 (5) |
| Nausea | 8 (5) | 2 (5) |
| Vomiting | 6 (4) | 0 |
| Nasopharyngitis | 9 (6) | 1 (2) |
| Hemoglobin, decreasedb | 15 (10) | 2 (5) |
| Hemoglobin, increasedb | 13 (8) | 2 (5) |
| Hypertension | 17 (11) | 8 (19) |
All data are given as n (%). DIALOGUE, DaIly orAL treatment increasing endOGenoUs Erythropoietin; TEAE, treatment-emergent adverse event.
Patients in the control group received placebo in DIALOGUE 1, continued darbepoetin treatment in DIALOGUE 2, and continued epoetin treatment in DIALOGUE 4.
As judged by the investigator.
Study drug-related serious TEAEs occurred in only one and two patients in the molidustat groups of DIALOGUE 2 and DIALOGUE 4, respectively (hyponatremia, prolonged QT interval, and hypotension). Five, four, and 11 serious AEs overall (three, two, and nine in the molidustat groups) were positively adjudicated in DIALOGUEs 1, 2, and 4, respectively.
Except for one report of a significant increase in alanine transaminase levels in the placebo group of DIALOGUE 1, no liver function-related AEs were reported during the studies.
There were generally no notable changes in laboratory parameters, with the exception of measures of iron metabolism. In DIALOGUE 1, mean ferritin and hepcidin values decreased over time in the molidustat groups, whereas mean total and unsaturated iron binding capacity increased (Supplemental Tables 9A and 10A). In the placebo group, iron metabolism parameters remained near baseline values, except for ferritin, which showed a transient increase. In DIALOGUE 2, there were mean reductions (between baseline and the end of treatment) in hepcidin and iron levels in the molidustat groups compared with an increase in the darbepoetin group (Supplemental Tables 9B and 10B). In DIALOGUE 4, mean changes in iron metabolism parameters were similar in the molidustat and epoetin groups (Supplemental Tables 9C and 10C).
Changes in LDL cholesterol between baseline and the end of treatment were small; in DIALOGUE 1: mean (SD) changes were 0.7 (28.9) mg/dl in the combined molidustat group and 3.3 (30.7) mg/dl in the placebo group. Mean (SD) changes in DIALOGUE 2 were 1.9 (24) mg/dl in the combined molidustat group and −1.3 (18.7) mg/dl in the darbepoetin group. In DIALOGUE 4, mean (SD) changes were −0.7 (22.3) mg/dl in the combined molidustat group and 0.2 (15.7) mg/dl in the epoetin group.
There were generally no notable changes in vital signs or electrocardiogram results in the three studies. Results of 24-hour ABPM indicated that molidustat does not increase BP. In DIALOGUE 1, molidustat had neither a beneficial nor a detrimental effect on BP relative to placebo, although the incidence of hypertension-related AEs was lower for molidustat (10%) than for placebo (25%).
Discussion
The global, phase 2 DIALOGUE program was designed to evaluate comprehensively the safety, efficacy, tolerability, pharmacokinetics, and pharmacodynamics of molidustat in patients with anemia of CKD, in individuals not on dialysis (DIALOGUE 1 and DIALOGUE 2) and in those on dialysis (DIALOGUE 4).
The fixed-dose, placebo-controlled study (DIALOGUE 1) demonstrated that molidustat provides increases in hemoglobin levels in patients not on dialysis. The observed plateau in hemoglobin levels at later time points is largely because of the discontinuation rate caused by high hemoglobin values. This should not be interpreted as a plateau of the mode of action, i.e., stimulation of erythropoiesis. No such plateau has been observed in preclinical experiments (20). Efficacy in patients not on dialysis was also demonstrated in DIALOGUE 2, in which treatment was switched from darbepoetin to molidustat or continued with darbepoetin. All molidustat dose arms were able to maintain the hemoglobin within the prespecified target range of 10.0–12.0 g/dl. The results, however, suggest that a molidustat starting dose of 25 or 50 mg once daily may be more appropriate in this patient population than 75 mg once daily, which appears to be associated with an increased likelihood of hemoglobin levels rising above prespecified limits compared with continued darbepoetin. This is consistent with the findings from DIALOGUE 1, in which more stopping events were caused by high hemoglobin levels in the molidustat 75 mg once daily group than in the 25 and 50 mg once daily groups.
In patients on dialysis (DIALOGUE 4), only molidustat starting doses of 75 and 150 mg once daily maintained hemoglobin levels within the target range after switching from epoetin. This was not unexpected, given the level of kidney function impairment of patients in this study. Indeed, although both kidney and hepatic erythropoietin production are affected, molidustat mainly addresses kidney erythropoietin production (20). In patients receiving dialysis treatment, switching from injectable ESA to molidustat 25 or 50 mg once daily resulted in an initial temporary drop in hemoglobin; understanding the underlying reasons for this would require further exploration in a future trial. Compared with the epoetin group, patients treated with molidustat starting doses of 75 or 150 mg once daily had lower response rates, spent less time within the target hemoglobin range, and were more likely to have hemoglobin levels above the prespecified limit. In both the DIALOGUE 2 and DIALOGUE 4 studies, the reduced time in the target hemoglobin range compared with darbepoetin/epoetin may reflect the inclusion criterion that therapy in the darbepoetin/epoetin groups was stable before the start of the study treatment period.
Several other HIF-PH inhibitors are in clinical development for anemia of CKD. These include vadadustat (AKB-6548), roxadustat (FG-4592), and daprodustat (GSK1278863). Published phase 2 studies in patients with CKD who are not on dialysis include a placebo-controlled study of vadadustat (23) and two studies of roxadustat (24,25), as well as a placebo-controlled study of daprodustat (26) and a dose-ranging study of daprodustat in both patients not on dialysis and those on hemodialysis (27); the results show that all agents increase and maintain hemoglobin levels in these patients. Two additional roxadustat studies (also phase 2) have been conducted in patients on dialysis (28,29). In one of these, patients taking epoetin had their treatment switched to roxadustat or continued on epoetin, and the results show that hemoglobin levels were maintained when patients were switched to the HIF-PH inhibitor (29). The above-mentioned phase 2 trials on daprodustat also enrolled patients on hemodialysis; in one trial, patients were on sufficient epoetin treatment before starting daprodustat, and the second trial enrolled epoetin-naïve patients. Hemoglobin was maintained in the switching study and it was increased in patients naïve to epoetin treatment.
Molidustat was generally well tolerated during the DIALOGUE studies. In DIALOGUE 2 and DIALOGUE 4, the increased incidence of TEAEs in the molidustat groups compared with that observed in the darbepoetin/epoetin groups was not unexpected, given that the studies were open label and that the darbepoetin/epoetin doses had been stabilized before the studies started. Of note, in the blinded DIALOGUE 1 trial, such imbalances were not seen.
The DIALOGUE studies were not powered to evaluate the signal for thromboembolic events compared with comparator ESAs; however, serious thromboembolic events were independently adjudicated. A few of these occurred in the molidustat treatment groups, but none were judged to be related to therapy by the investigator (Supplemental Table 11). Another recognized side effect of ESA therapy is hypertension (30). Data from these studies indicate that BP, which was measured using 24-hour ABPM, was not increased by molidustat; in fact, there was some suggestion that molidustat treatment may slightly decrease BP, although the small patient numbers preclude firm conclusions.
The DIALOGUE studies have several limitations. These include low patient numbers per treatment group and a relatively short duration of treatment contributing to the changes in hemoglobin levels observed after treatment initiation; however, this is customary for phase 2 clinical studies, which serve to provide data from which to design larger, longer phase 3 trials. Other design elements of the DIALOGUE 1 study were robustness, as it included a placebo arm and was double-blinded, whereas DIALOGUE 2 and DIALOGUE 4 were open label, which may have introduced bias (e.g., in AE reporting). Discontinuation rates in all three studies were relatively high. However, for DIALOGUE 1, this mainly reflects the inclusion of stopping rules in the event of low/high hemoglobin levels as well as exaggerated hemoglobin increase, designed to safeguard patients in the study because dose modifications were not permitted. In DIALOGUE 2 and DIALOGUE 4, completion rates in patients treated with molidustat were lower than patients treated with darbepoetin or epoetin, but again, this likely reflects the fact that ESA doses were stable before patients entered the study.
In conclusion, results of these phase 2b studies indicate that the overall efficacy/safety profile of molidustat enables further development in both patients with CKD who are not on dialysis and those with CKD who are on dialysis. Hemoglobin levels can be maintained after switching from ESAs to a flexible dose of molidustat, with manageable side effects. No serious thromboembolic events were related to treatment. Continued evaluation of molidustat in larger phase 3 studies is warranted.
Disclosures
I.C.M. received research funding for the DIALOGUE studies, honoraria for steering committee activities, and speaker fees from Bayer. He has also received research support and speaker’s honoraria from Akebia, Astellas, FibroGen, and GlaxoSmithKline. T.A. received consulting fees from Astellas, Bayer Health Care, GlaxoSmithKline, JT Pharmaceuticals, Kissei Pharmaceutical Co. Ltd., Kyowa Hakko Kirin, Nipro Corporation, Fuso Pharmaceutical Industries Ltd., and Ono Pharmaceutical Co. Ltd., and lecture fees from Bayer Health Care, Chugai Pharmaceutical Co. Ltd., Kyowa Hakko Kirin, and Torii Pharmaceutical Co. Ltd. J.S.B. served on the steering committees for the DIALOGUE studies and for an Amgen-sponsored darbepoetin clinical trial. T.B. and T.K. are employees of Bayer Pharma AG.
Supplementary Material
Acknowledgments
Medical writing support was provided by Jesse Alderson of Oxford PharmaGenesis, Oxford, UK, with funding from Bayer AG. Statistical analysis and interpretation of results was supported by Gerald Staedtler, Bayer AG.
Availability of the data underlying this publication will be determined according to Bayer’s commitment to the European Federation of Pharmaceutical Industries and Associations/Pharmaceutical Research and Manufacturers of America “Principles for responsible clinical trial data sharing.” This pertains to scope, time point, and process of data access. As such, Bayer commits to sharing upon request from qualified scientific and medical researchers patient-level clinical trial data, study-level clinical trial data, and protocols from clinical trials in patients for medicines and indications approved in the United States and European Union as necessary for conducting legitimate research. This applies to data on new medicines and indications that have been approved by the European Union and United States regulatory agencies on or after January 1, 2014.
Interested researchers can use www.clinicalstudydatarequest.com to request access to anonymized patient-level data and supporting documents from clinical studies to conduct further research that can help advance medical science or improve patient care. Information on the Bayer criteria for listing studies and other relevant information is provided in the study sponsors section of the portal. Data access will be granted to anonymized patient-level data, protocols, and clinical study reports after approval by an independent scientific review panel. Bayer is not involved in the decisions made by the independent review panel. Bayer will take all necessary measures to ensure that patient privacy is safeguarded.
These studies were funded by Bayer AG.
Study concept, protocol, and statistical analysis plan as well as analyses were performed on the basis of recommendations by both the steering committee and the sponsor. Interpretations were made by the independent steering committee (authors of the manuscript) only.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02510218/-/DCSupplemental.
Supplemental Material
Supplemental Table 1. Key inclusion and exclusion criteria for DIALOGUEs 1, 2, and 4.
Supplemental Table 2. Daily average dosages of molidustat in (A) DIALOGUE 2 and (B) DIALOGUE 4.
Supplemental Table 3. Secondary efficacy variables in the DIALOGUE program 10.
Supplemental Table 4. Baseline demographics and clinical characteristics in DIALOGUEs 1, 2, and 4.
Supplemental Table 5. Changes from baseline in mean local hemoglobin at evaluation period by starting dose in DIALOGUE 1, DIALOGUE 2, and DIALOGUE 4 (last observation carry forward).
Supplemental Table 6. Time within the hemoglobin range during treatment with molidustat and active treatment in DIALOGUE 2 and DIALOGUE 4.
Supplemental Table 7. Patients receiving red blood cell transfusion and ESA treatment for low hemoglobin as rescue treatment during the study by study and starting dose.
Supplemental Table 8. TEAEs reported in >10% of patients in any group in DIALOGUEs 1, 2, and 4 by molidustat dose group.
Supplemental Table 9. Baseline values of measures of iron metabolism in DIALOGUE 1, DIALOGUE 2, and DIALOGUE 4.
Supplemental Table 10. Changes in measures of iron metabolism between baseline and end of treatment in DIALOGUE 1, DIALOGUE 2, and DIALOGUE 4.
Supplemental Table 11. Patients with thromboembolic events in any group in DIALOGUEs 1, 2, and 4 by molidustat dose group.
Supplemental Figure 1. Study design of (A) DIALOGUE 1, (B) DIALOGUE 2, and (C) DIALOGUE 4. Molidustat doses were suspended if Hb was >13.0 g/dl or if the rise in Hb was >1.0 g/dl in 2 weeks. Participants who required a dose suspension for ≥6 consecutive weeks had to be withdrawn from the study.
Supplemental Figure 2. Dose titration scheme for DIALOGUEs 2 and 4.
References
- 1.Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR: Impact of anemia on hospitalization and mortality in older adults. Blood 107: 3841–3846, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Hsu CY, McCulloch CE, Curhan GC: Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: Results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 13: 504–510, 2002 [DOI] [PubMed] [Google Scholar]
- 3.Astor BC, Muntner P, Levin A, Eustace JA, Coresh J: Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988-1994). Arch Intern Med 162: 1401–1408, 2002 [DOI] [PubMed] [Google Scholar]
- 4.El-Achkar TM, Ohmit SE, McCullough PA, Crook ED, Brown WW, Grimm R, Bakris GL, Keane WF, Flack JM; Kidney Early Evaluation Program : Higher prevalence of anemia with diabetes mellitus in moderate kidney insufficiency: The Kidney Early Evaluation Program. Kidney Int 67: 1483–1488, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Babitt JL, Lin HY: Mechanisms of anemia in CKD. J Am Soc Nephrol 23: 1631–1634, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vos FE, Schollum JB, Coulter CV, Doyle TC, Duffull SB, Walker RJ: Red blood cell survival in long-term dialysis patients. Am J Kidney Dis 58: 591–598, 2011 [DOI] [PubMed] [Google Scholar]
- 7.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group : Clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279–335, 2012 [Google Scholar]
- 8.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 9.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D; CHOIR Investigators : Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A; CREATE Investigators : Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators : A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, de Zeeuw D, Ivanovich P, Levey AS, Parfrey P, Remuzzi G, Singh AK, Toto R, Huang F, Rossert J, McMurray JJ, Pfeffer MA; Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Investigators : Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 363: 1146–1155, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK: Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 74: 791–798, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unger EF, Thompson AM, Blank MJ, Temple R: Erythropoiesis-stimulating agents--time for a reevaluation. N Engl J Med 362: 189–192, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Krapf R, Hulter HN: Arterial hypertension induced by erythropoietin and erythropoiesis-stimulating agents (ESA). Clin J Am Soc Nephrol 4: 470–480, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Luo J, Jensen DE, Maroni BJ, Brunelli SM: Spectrum and burden of erythropoiesis-stimulating agent hyporesponsiveness among contemporary hemodialysis patients. Am J Kidney Dis 68: 763–771, 2016 [DOI] [PubMed] [Google Scholar]
- 17.Gilbertson DT, Peng Y, Arneson TJ, Dunning S, Collins AJ: Comparison of methodologies to define hemodialysis patients hyporesponsive to epoetin and impact on counts and characteristics. BMC Nephrol 14: 44, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossert J, Gassmann-Mayer C, Frei D, McClellan W: Prevalence and predictors of epoetin hyporesponsiveness in chronic kidney disease patients. Nephrol Dial Transplant 22: 794–800, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Koury MJ, Haase VH: Anaemia in kidney disease: Harnessing hypoxia responses for therapy. Nat Rev Nephrol 11: 394–410, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flamme I, Oehme F, Ellinghaus P, Jeske M, Keldenich J, Thuss U: Mimicking hypoxia to treat anemia: HIF-stabilizer BAY 85-3934 (Molidustat) stimulates erythropoietin production without hypertensive effects. PLoS One 9: e111838, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schofield CJ, Ratcliffe PJ: Oxygen sensing by HIF hydroxylases. Nat Rev Mol Cell Biol 5: 343–354, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Wenger RH, Hoogewijs D: Regulated oxygen sensing by protein hydroxylation in renal erythropoietin-producing cells. Am J Physiol Renal Physiol 298: F1287–F1296, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Pergola PE, Spinowitz BS, Hartman CS, Maroni BJ, Haase VH: Vadadustat, a novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int 90: 1115–1122, 2016 [DOI] [PubMed] [Google Scholar]
- 24.Besarab A, Provenzano R, Hertel J, Zabaneh R, Klaus SJ, Lee T, Leong R, Hemmerich S, Yu KH, Neff TB: Randomized placebo-controlled dose-ranging and pharmacodynamics study of roxadustat (FG-4592) to treat anemia in nondialysis-dependent chronic kidney disease (NDD-CKD) patients. Nephrol Dial Transplant 30: 1665–1673, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Provenzano R, Besarab A, Sun CH, Diamond SA, Durham JH, Cangiano JL, Aiello JR, Novak JE, Lee T, Leong R, Roberts BK, Saikali KG, Hemmerich S, Szczech LA, Yu KH, Neff TB: Oral hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat (FG-4592) for the treatment of anemia in patients with CKD. Clin J Am Soc Nephrol 11: 982–991, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holdstock L, Meadowcroft AM, Maier R, Johnson BM, Jones D, Rastogi A, Zeig S, Lepore JJ, Cobitz AR: Four-week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol 27: 1234–1244, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brigandi RA, Johnson B, Oei C, Westerman M, Olbina G, de Zoysa J, Roger SD, Sahay M, Cross N, McMahon L, Guptha V, Smolyarchuk EA, Singh N, Russ SF, Kumar S; PHI112844 Investigators : A novel hypoxia-inducible factor-prolyl hydroxylase inhibitor (GSK1278863) for anemia in CKD: A 28-day, phase 2A randomized trial. Am J Kidney Dis 67: 861–871, 2016 [DOI] [PubMed] [Google Scholar]
- 28.Besarab A, Chernyavskaya E, Motylev I, Shutov E, Kumbar LM, Gurevich K, Chan DT, Leong R, Poole L, Zhong M, Saikali KG, Franco M, Hemmerich S, Yu KH, Neff TB: Roxadustat (FG-4592): Correction of anemia in incident dialysis patients. J Am Soc Nephrol 27: 1225–1233, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Provenzano R, Besarab A, Wright S, Dua S, Zeig S, Nguyen P, Poole L, Saikali KG, Saha G, Hemmerich S, Szczech L, Yu KH, Neff TB: Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: A phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis 67: 912–924, 2016 [DOI] [PubMed] [Google Scholar]
- 30.Palmer SC, Saglimbene V, Mavridis D, Salanti G, Craig JC, Tonelli M, Wiebe N, Strippoli GF: Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: A network meta-analysis. Cochrane Database Syst Rev (12): CD010590, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994 [DOI] [PubMed] [Google Scholar]
- 32.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A; Collaborators developing the Japanese equation for estimated GFR : Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 53: 982–992, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.