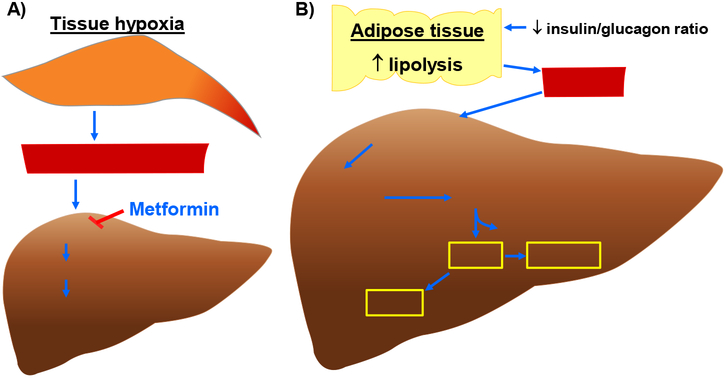
Fig. 2.
Molecular mechanisms that contribute to enhanced blood lactate and ketone body levels. A) Increase in blood lactate level (and thereby lactic acidosis) in hypoxic or metformin-treated conditions. A decrease in microcirculatory perfusion in target tissues (e.g., skeletal muscle) would result in tissue hypoxia. An increase in anaerobic glycolysis under hypoxic conditions would lead to enhanced lactate formation/release into the systemic circulation. In addition, lactate accumulation may result from its impaired hepatic clearance due to high concentration of plasma metformin, which would inhibit utilization of lactate toward hepatic gluconeogenesis. B) Synthesis of ketone bodies (e.g., βOHB, AcAc, and acetone) in DKA. A decrease in systemic insulin-to-glucagon ratio would promote lipolysis in adipose tissue to enhance FFA release and its uptake by the liver. In the hepatic mitochondria, HMGCS2 catalyzes the condensation reaction between AcAc-CoA and Acetyl-CoA (derived from β-oxidation of fatty acids) to form HMG-CoA. Subsequently, HMGCL cleaves HMG-CoA to generate AcAc. In the presence of increased NADH/NAD+ ratio, BDH1 catalyzes the conversion of AcAc to βOHB. In addition, AcAc undergoes nonenzymatic decarboxylation to form acetone. AcAc, acetoacetate; AcAc-CoA, acetoacetyl-CoA; BDH1, mitochondrial βOHB dehydrogenase; FFA, free fatty acids; HMG-CoA, 3-hydroxymethylglutaryl-CoA; HMGCL, HMG-CoA lyase; HMGCS2, mitochondrial HMG-CoA synthase.