Abstract
Introduction:
In general, mice develop chronic and non-healing periapical lesions following endodontic infection. We recently found that, surprisingly, TLR2/IL-10 double KO (dKO) mice exhibited acute but resolving osteomyelitis-like inflammation. In this study, we examined the kinetics of endodontic infection-induced inflammation in TLR2/IL-10-dKO mice and explored a potential mechanism of periapical wound healing mediated by hypoxia-inducible factor 1 alpha subunit (HIF-1α) and arginase-1.
Methods:
TLR2/IL-10-dKO and wild-type (WT) C57BL/6J mice were subjected to endodontic infection in the mandibular first molars. Mice were sacrificed on days 0 (non-infected), 10 and 21 post-infection. The extent of bone destruction, inflammation, bone deposition, and gene expression were determined by μCT, histology, bone polychrome labeling, and microarray analysis. In addition, the effect of blocking endogenous HIF-1α was tested in infected TLR2/IL-10-dKO mice using the specific inhibitor YC-1.
Results:
Infected TLR2/IL-10-dKO mice exhibited extensive bone destruction and inflammation on day 10, followed by spontaneous periapical wound healing including bone formation and resolution of inflammation by day 21 post-infection. In contrast, WT mice developed increasing chronic periapical inflammation over the 21-day observation period. Gene expression analyses and immunohistochemistry revealed that HIF-1α and arginase-1 were upregulated in the spontaneous wound healing in TLR2/IL-10-dKO mice. Blocking of HIF-1α in TLR2/IL-10-dKO mice using YC-1 resulted in significant inhibition of regenerative bone formation.
Conclusion:
The TLR2/IL-10-dKO mouse is a novel model resembling osteomyelitis of the jaws, in which HIF-1α and arginase-1 appear to be crucial factors in spontaneous wound healing and bone repair.
Keywords: osteomyelitis, mouse model, hypoxia-inducible factor 1, arginase-1, wound healing
Introduction
Osteomyelitis is a bacterial infection that results in inflammation of the bone marrow and adjacent bone. Acute osteomyelitis of the jaws is most commonly caused by an exacerbation of an untreated periapical lesion/abscess, resulting in rapid and severe tissue and bone destruction (1). In contrast, chronic osteomyelitis is a sequel to acute osteomyelitis or occasionally occurs in response to a low-grade inflammatory process, leading to trabecular bone formation and a reduction of the marrow spaces (1).
Besides infection by virulent bacteria, susceptibility to osteomyelitis is modulated by various genetic factors including interleukin (IL)-1 alpha (−889) promoter polymorphisms (2), deficiency of IL-1 receptor antagonist (IL-1RA) (3), reduced Il10 promotor phosphorylation (4), reduced myeloid IL-10 expression (4), and dysfunction of Toll-like receptor 2 (TLR2) (5). In previous studies, the development of infection-induced bone loss and inflammation were elevated in IL-1RA-deficient (KO), IL-10-KO, and TLR2-KO mice (6–8), but did not progress to osteomyelitis. These findings suggest that the host has evolved endogenous multi-layer defense mechanisms for preventing severe infectious/inflammatory diseases in bone.
Recently, we generated a TLR2/IL-10-double deficient (dKO) mouse that uniquely develops infection-induced inflammation resembling the clinical course of osteomyelitis of the jaws. In the present study, we characterize the pathogenesis of osteomyelitis-like inflammation in this model, and define several potential mechanisms involved in reactive bone formation and spontaneous wound healing.
Materials and Methods
Animals
TLR2/IL-10-double knockout (dKO), generated by cross-breeding of TLR2-KO and IL-10-KO (The Jackson Laboratory, Bar Harbor, ME), and wild-type C57BL/6J (WT) mice were employed. A total of 19 TLR2/IL-10-dKO and 11 WT mice were used. All protocols were approved by The Forsyth Institutional Animal Care and Use Committee.
Endodontic infection
Exposed mandibular first molar pulps of 5–6 week old mice were inoculated with Parvimonas micra (ATCC 33270), Streptococcus intermedius (ATCC 27335), Prevotella intermedia (ATCC 25611), and Fusobacterium nucleatum (ATCC 25586) as described (7,8).
Micro computed tomography (μCT) and histology
On days 10 and 21 post-infection, mice were sacrificed by CO2 inhalation. Mandibles were isolated and hemisected. One hemimandible was fixed and subjected to μCT analysis (7,9) followed by histology including immunohistochemistry for arginase-1 (Arg1) (sc-18351 (N20), Santa Cruz Biotechnology, dilution 1:50), macrophages (anti-F4/80 12310, BioLegend, dilution 1:100), inducible nitric oxide synthase (iNOS) (ab3523, Abcam, dilution 1:400); and HIF-1α (AVARP 20009_P050, Aviva Systems Biology, dilution 1:200). Primary antibodies were detected using Vector ELITE ABC kits (Vector Laboratories) according to the manufacturer’s instructions.
Direct polychrome bone labeling
Bone deposition in each strain (n=3) was evaluated using calcein blue, xylenol orange, calcein, alizarin complex one, and hematoporphyrin (all from Sigma-Aldrich (St. Louis, MO)) (10). Mice were injected intraperitoneally on days −1, 6, 9, 12, and 15 relative to pulpal infection, and were killed on day 18. Polished epoxy resin-embedded mandible samples (EMbed 812; Electron Microscopy Sciences, Hatfield, PA) were analyzed using a Zeiss Stemi SV11 microscope.
Microarray and real-time RT-PCR
For analysis on Affymetrix GeneChip™ Mouse Gene 1.1 ST Array (Thermo Fisher Scientific Inc., Waltham, MA), total RNA samples were isolated from periapical lesions in hemimandibles (8) and were subjected to fragmented/labeled cDNA synthesis using the Ovation Pico WTA System, WT-Ovation Exon Module, and the Encore® Biotin Module (all NuGEN Technologies Inc., San Carlos, CA) following manufacturer’s instructions. Changes in gene expression were considered significant if the detection P value was < 0.05 and the change-folds value was >2.0. The infection effect on gene expression profiles in each strain was assessed by either one-way ANOVA or Fisher’s exact t-test using the Partek Genomic suite V6 (Partek, St. Lois, MO). The expression of Hif1a, Cp (ceruloplasmin), and Caix (calbonic anhydrase 9) was further validated by real-time RT-PCR using the 2−ΔΔCT method and Student’s t-test. Ywhaz (14-3-3 protein zeta/delta) served as a reference gene. The list of primers is shown in Supplemental Materials.
Inhibition of HIF activation in vivo
The specific HIF inhibitor YC-1 (Sigma-Aldrich) was locally injected in infected TLR2/IL-10-dKO mice via root canals (5 μg/kg/time) using a 36G needle at 2-day intervals from days 1 to 19 post-infection (11,12). Mice receiving PBS served as controls. The status of reactive bone formation was evaluated on day 21 using μCT.
Results
TLR2/IL-10-dKO mice develop osteomyelitis-like inflammation and subsequent spontaneous wound healing
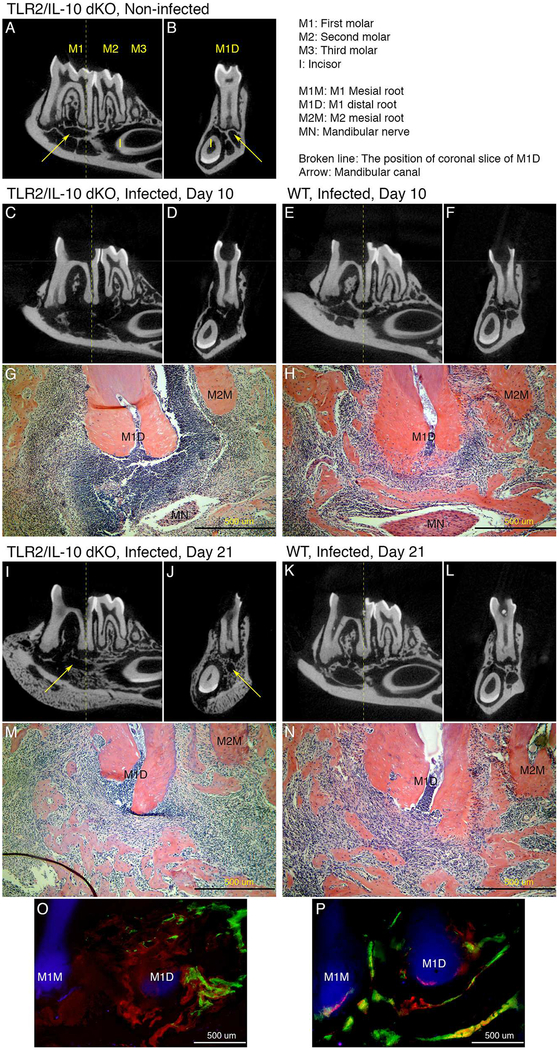
TLR2/IL-10-dKO and WT mice were subjected to pulpal infection. On day 10 post-infection, TLR2/IL-10-dKO mice exhibited severe bone destruction compared to WT controls (Fig. 1A–1H). Histology revealed severe tissue destruction and extreme inflammatory cell infiltration in TLR2/IL-10-dKO mice, resembling acute osteomyelitis (Fig. 1G). In contrast, WT mice exhibited localized mild-moderate inflammatory cell infiltration (Fig. 1H). Surprisingly, on day 21, TLR2/IL-10-dKO mice developed a sunburst appearance of mandibular bone by μCT, indicating a periosteal reaction (Fig. 1I, 1J), and endosteal reactive bone formation (Fig. 1I, 1J). Furthermore, the extreme inflammatory cell infiltration seen in TLR2/IL-10-dKO-day 10 lesions spontaneously resolved almost completely on day 21 (Fig. 1M). In contrast, WT developed radicular granulomas with moderate inflammatory cell infiltration (Fig. 1N), and a moth-eaten appearance of residual bone (Fig. 1K, 1L) with no apparent bone formation. See Supplemental Materials for clinical signs of illness.
Figure 1. Histopathology of periapical lesions in TLR2/IL-10-dKO and WT mice.
(A and B) The observation periods of days 0, 10 and, 21 correspond to non-infected control, acute phase, and chronic phase, respectively. μCT images of the mandibular molar region in non-infected dKO on the sagittal (A) and coronal plane at the center of the first molar distal root (B). (C and D) μCT images of mandibular molar region of the dKO on day 10 post infection. Sagittal plane (C); coronal plane (D). Notably altered intraosseous structure (including trabecular bone, interradicular septum, mandibular canal, and socket of the mandibular incisor), thinned cortical bone, and periosteal reaction (new bone formation at the bottom of mandible) were observed (n=4). (E and F) μCT images of mandibular molar region of WT on day 10 on the sagittal E) and coronal plane (F). Although minimal bone destruction was seen, the bony structure was generally conserved in WT (n=4). (G and H) H&E staining of the periapical region on day 10 in dKO (G) and WT (H). Periapical abscess with extensive tissue destruction and remarkable inflammatory cell infiltration was present in dKO mice. In contrast, WT mice exhibited only mild inflammation centered around the apical foramen (20X magnification; Scale bar: 500 μm). (I and J) μCT images of dKO on day 21 on the sagittal (I) and coronal plane (J). Considerable reactive endosteal and periosteal bone formation occurred, resulting in reconstruction of the mandibular canal and the incisor socket (n=4). (K and L) μCT images of WT on day 21on the sagittal (K) and coronal plane (L). Periapical bone destruction was progressive compared to day 10 (n=4). (M and N) H&E staining of the periapical region on day 21 in the dKO (M) and WT (N). Substantial spontaneous resolution of inflammatory cell infiltration and bone formation was observed in dKO mice. Periodontal ligament tissue between M1D and M2M was undergoing repair. In contrast, mild to moderate inflammation continued in WT (20X; Scale bar: 500 μm). (O and P) Representative images indicating the kinetics of bone deposition in TLR2/IL-10-dKO (O) and WT mice (P) (n=3/strain). Calcein blue (blue), xylenol orange (orange), calcein (green), alizarin complex one (dark red), and hematoporphyrin (bright red) were injected i.p. on days −1, 6, 9, 12, and 15 relative to pulpal infection. In the area surrounding the mandibular first molar, the bone deposited on day −1 (blue) was already gone at day 18 in both dKO and WT mice due to pathological and physiological resorption. In dKO mice (O), most of bone deposited before day 9 (blue, orange, and green) did not exist around the first molar. Instead, the space was occupied with irregular-shaped bone deposited in days 12 (dark red) and 15 (bright red). In WT mice (P), intra-bony structure mainly consisted of the bone deposited on days 9 (green), 12 (dark red), and 15 (bright red) along the bone deposited on day 6 (orange). MR: mesial root, first molar, DR: distal root, first molar, Scale bar: 500 μm, 20X magnification.
Reactive bone formation in TLR2/IL-10-dKO and WT mice
The total bone volume in TLR2/IL-10-dKO mandibles decreased dramatically to ≃25% on day 10 compared to non-infected controls (Supplemental Fig. 1), with a striking reversal on day 21(≃120% vs. controls). In contrast, ≃5% reduction of total bone volume occurred in WT during the experiment. The kinetics of bone deposition was further analyzed using dynamic labelling (Fig. 1O, 1P). Extensive bone deposition, which was not associated with the residual bone, occurred around periapices in TLR2/IL-10-dKO between days 9 and 18, although the shape and location of newly deposited bones were irregular (Fig. 1I, 1J, 1M, 1O). In contrast, the deposition in WT occurred solely along the residual bone surfaces.
Resolution of inflammation in TLR2/IL-10-dKO mice
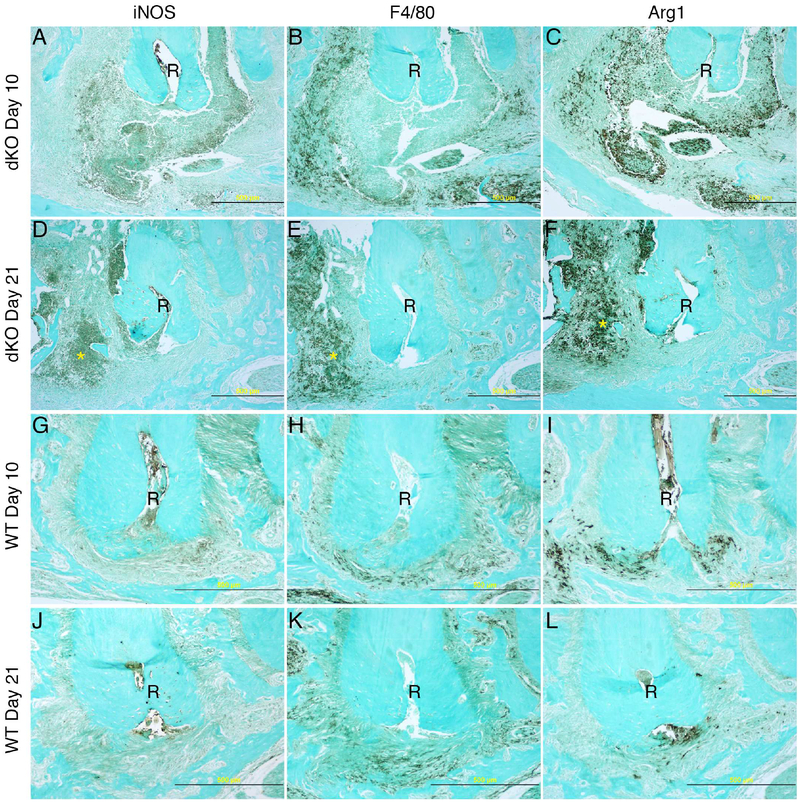
On day 10 post-infection, proinflammatory iNOS(+) cells occupied the core area of abscesses in TLR2/IL-10-dKO mice (Fig. 2A), with the core surrounded by F4/80(+) cells (Fig. 2B). Interestingly substantial numbers of arginase-1 (Arg1)(+) cells accumulated simultaneously in the core region (Fig. 2C). Arg1(+) cells may consist of both F4/80(−) and F4/80(+) leukocytes. On day 21 (Fig. 2D–2F), dramatic resolution of the core with considerable reduction of Arg1(+) cells was observed in TLR2/IL-10-dKO mice. In an area adjacent to the interradicular septum (asterisk), iNOS(+), F4/80(+), and Arg1(+) cells still co-existed, indicating a state of chronic inflammation in TLR2/IL-10-dKO mice. In WT lesions, mild to moderate diffuse infiltration of iNOS(+), F4/80(+), and Arg1(+) cells was observed on day 10 (Fig. 2G–2I), which persisted to day 21 (Fig. 1N; Fig. 2J–2L), indicating a progressive chronic inflammatory state in WT lesions.
Figure 2. Expression of iNOS, F4/80, and Arg1 in periapical lesions in TLR2/IL-10-dKO and WT mice.
Serial sections of the samples presented in Fig. 1 were subjected to immunohistochemistry. iNOS: inflammatory marker expressed by pro-inflammatory cells including neutrophils and M1 macrophages. F4/80: total macrophages. Arg1: pro-resolving M2 macrophages and wound healing. (A - C) TLR2/IL-10-dKO lesions on day 10 post infection. (D - F) TLR2/IL-10-dKO lesions on day 21. (G - I) WT lesions on day 10. (J - L) WT lesion on day 21. R: first molar distal root; 20× magnification. Scale bar: 500 μm. Note that panels G to L were framed to emphasize the status of immunostaining-positive cells in WT periapical lesions.
Gene expression profile on day 10 post-infection
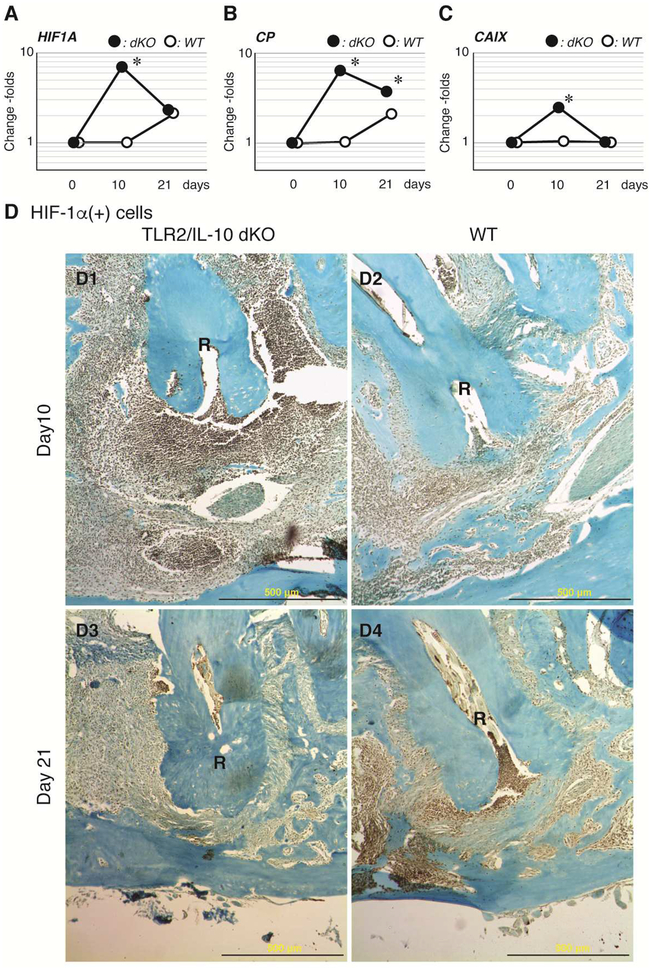
We compared periapical gene expression profiles on days 0 (baseline) and 10 post-infection in each strain. The list of the top 25 up-regulated genes in TLR2/IL-10-dKO and WT mice is summarized in Supplemental Tables 1 and 2, respectively. Hif1a, which regulates bone formation and inflammation (13,14), was strikingly up-regulated in TLR2/IL-10-dKO lesions. In qRT-PCR analysis, the expression of Hif1a in TLR2/IL-10-dKO peaked on day 10 and declined thereafter along with the spontaneous resolution of inflammation (Fig. 3A). In WT, the Hif1a gene was modestly elevated on day 21 (p>0.05). The activation of the HIF pathway in TLR2/IL-10-dKO-day 10 lesions was confirmed by up-regulation of Cp and Caix, which are downstream genes induced by HIF-1 (Fig. 3B, 3C). In contrast, Hif1a and Cp were belatedly up-regulated in WT-day 21 lesions. On the other hand, genes related to pro-resolving M2 macrophage activation, Arg1 and Il13ra1, were significantly elevated only in the TLR2/IL-10-dKO lesions, but not in WT on day 10 (see Fig. 2 for Arg1 protein expression in situ).
Figure 3. Expression kinetics of HIF-1α in periapical lesions.
qRT-PCR was employed to compare gene expression of Hif1a (A) and its downstream genes Cp (B) and Caix (C) in TLR2/IL-10 KO and WT lesions. Y-axis: logarithmic scale. Closed dot: dKO, Open dot: WT, *: P < 0.05 vs. corresponding day 0 control. (D) HIF-1α protein expression was validated by immunohistochemistry on serial sections of the samples presented in Fig. 1 and 2. (D1) TLR2/IL-10-dKO lesion on day 10 after pulpal infection. (D2) WT lesion on day 10. HIF-1α was faintly expressed in cells around the apical foramen. (D3) TLR2/IL-10-dKO lesion on day 21. (D4) WT lesion on day 21. R: distal root, first molar, Scale bar: 500 μm, 20X magnification.
Distinct kinetics of HIF-1α (+) cells in TLR2/IL-10-dKO and WT mice
The high expression of HIF-1α protein was confirmed in infiltrating cells in TLR2/IL-10-dKO-day 10 lesions (Fig. 3D1). In contrast, staining for HIF-1α protein was weak in WT-day 10 lesions (Fig. 3D2). On day 21, most HIF-1α (+) cells disappeared from the periapical regions in TLR2/IL-10-dKO mice consistent with the resolution of inflammation (Fig. 3D3). In WT lesions, HIF-1α was evident in the area of inflammatory cell infiltration on day 21 (Fig. 3D4).
Effect of inhibition of the HIF-1α pathway on endosteal reactions in TLR2/IL-10-dKO mice
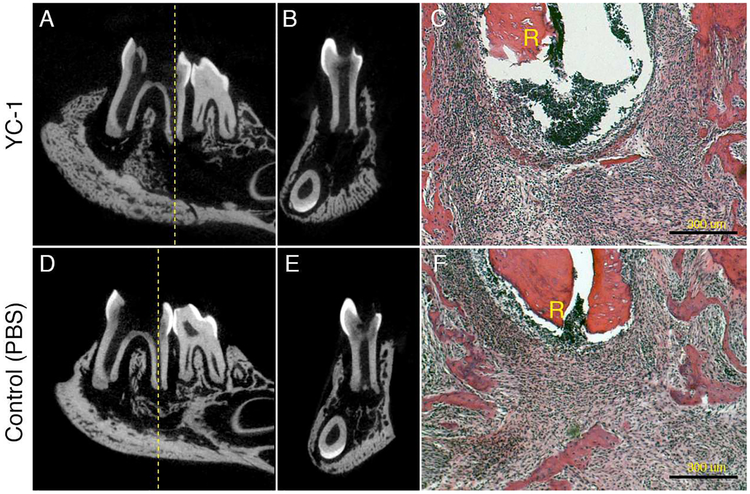
Our findings above suggested that activation of HIF-1α is involved in the spontaneous healing and resolution of acute inflammation. To test this hypothesis, we administered YC-1, a well characterized HIF-1α inhibitor, to infected TLR2/IL-10-dKO mice locally via the root canals from days 1 to 19 post-infection. As shown in Fig. 4A, 4B, 4D, and 4E, YC-1 prevented reactive bone formation in the periapical region on day 21, indicating that activation of HIF-1α was crucial for the bone repair. The level of inflammation was also reduced following YC-1 treatment vs. controls (Fig. 4C, 4F).
Figure 4. Effect of YC-1 on wound healing in TLR2/IL-10-dKO mice.
The effect of YC-1-mediated HIF-1 inhibition on wound healing in infected TLR2/IL-10-dKO mice was determined on day 21 post pulpal infection (n=4/group). YC-1-mediated inactivation of the HIF-1α pathway in this model was confirmed by 69–83% suppression of CP gene expression vs. control. (A and B) Mandibular molar region in YC-1-treated TLR2/IL-10-dKO on the sagittal (A) and, coronal plane. Broken line: center of first molar distal root. (C) H&E staining of YC-1 treated TLR2/IL-10-dKO. R: distal root, Scale bar: 300 μm (20X). (D and E) PBS-injected TLR2/IL-10-dKO control, on the sagittal (D) and coronal plane. (F) H&E staining of PBS treated TLR2/IL-10-dKO. In all samples, the level of inflammation was milder in YC-1-treated mice compared to controls. R: first molar distal root, Scale bar: 300 μm, 20X magnification.
Discussion
To date, no animal models of endodontic infection-induced osteomyelitis of the jaws have been reported, limiting our knowledge of the host factors that affect the onset and the course of osteomyelitis. In this study we have characterized the pathogenesis of osteomyelitis-like inflammation caused by endodontic infection in TLR2/IL-10-dKO mice. TLR2/IL-10-dKO mice exhibited rapid progression of osteolytic acute inflammation within 10 days post-infection. This extreme phenotype is most likely dependent on the additive or synergistic effect of hyperinflammatory phenotypes in TLR2-KO and IL-10-KO strains (7,8). However, TLR2/IL-10-dKO mice also surprisingly underwent spontaneous resolution of inflammation with reparative bone formation by day 21. Such wound healing has never been observed in WT, TLR2-KO or IL-10-KO parental strains, although new bone formation does occur after approximately 2 months coincident with lesion stabilization (15).
Microarray analysis suggested that Hif1a and Arg1 were involved in the process of spontaneous wound healing. HIF-1 is a master regulator expressed in response to hypoxia. HIF-1 is a heterodimeric transcription factor consisting of an alpha and a beta subunit. HIF-1α is unstable under normoxia due to prorylhydoroxylase-mediated ubiquitination and following proteasomal degradation. Hypoxia stabilizes HIF-α and leads to dimerization with HIF-1β, activating HIF-1 inducible pathways (16). The primary role of activated HIF is to maintain an adequate oxygen supply to tissues. We speculate that elevated oxygen consumption by intensely infiltrated inflammatory cells led to extreme hypoxia and subsequent HIF-1 activation in TLR2/IL-10-dKO mice (Fig. 1G, 2A–2C, and 3).
Activation of HIF was confirmed by up-regulation of downstream targets Cp, Caix, iNOS, and Arg1. Of those, Cp is known to contribute to localized aggressive periodontitis through priming of neutrophils (17). In addition, nitric oxide generated by iNOS is critical in IL-1-stimulated bone destruction via RANKL, which is a central mechanism of periapical bone destruction (18). Significant up-regulation of Cp and iNOS in TLR2/IL-10-dKO mice may thus represent tissue destructive pathways induced by HIF-1. In contrast, Arg1 interrupts iNOS-mediated responses by competing for their common substrate, L-arginine (19). Therefore, potent induction of Arg1 in TLR2/IL-10-dKO mice may represent a key anti-inflammatory/pro-resolving mechanism of HIF-1 in osteomyelitis-like inflammation.
Macrophages are important in periapical lesion development. iNOS and Arg1 are representative biomarkers of pro-inflammatory M1 and pro-resolving/healing M2 macrophages, respectively. M2 macrophages assume a key role in efferocytosis and resolution of inflammation (20). Interestingly, HIF-alpha subunits (HIF-1α and HIF-2α) affect polarization of M1/M2 macrophages via NO homeostasis mediated by iNOS and Arg1 (19,21). This regulatory system (so-called HIF-alpha switching) requires further investigation as a putative mechanism in the accumulation of M2 macrophage in the abscess core (Fig. 2C). We observed heterogeneity of Arg1(+) cells in the abscess (Fig. 2A–2C), which may reflect the presence of myeloid-derived suppressor cells (MDSC). In the tumor microenvironment, activation of HIF-1 promotes differentiation of MDSC to M2/tumor-associated macrophages (21,22). Although definitive cell phenotyping is required for validation, it is likely that MDSC represent another player in the network regulating resolution of inflammation and wound healing in this model (22).
Activation of HIF-1 is essential in repair and regeneration of damaged bone via angiogenic-osteogenic coupling (23). In a distraction osteogenesis model, bone regeneration is mediated by HIF-1-activation and subsequent induction of VEGF. Indeed, deferoxamine-mediated HIF-1-activation results in accelerated healing including denser and blood vessel-rich woven bone formation. HIF-1 deletion in osteoblasts leads to reduced angiogenesis and delayed bone regeneration (13). HIF-1-induced CXCL12 appears to be important in the trafficking and recruitment of mesenchymal stem cells/osteoblast progenitor cells to the site of injury in this process (24,25). In TLR2/IL-10-dKO mice, up-regulation of Cxcl12 (Supplemental Table 1) and Vegfa (p=0.002 vs. baseline) was confirmed by microarray. This strain also exhibits potent woven bone formation as reactive bone formations on day 21 post-infection (Fig. 1I, 1J). Collectively, angiogenic-osteogenic coupling appears to be elevated in this model. In contrast, local activation of HIF-1α failed to induce VEGF and bone formation in WT periapical lesions (12). As noted, infected TLR2-KO mice exhibit active angiogenesis, but no periapical bone repair occurs (8). For improved healing, further investigation of the conditions under which HIF-1-mediated angiogenic-osteogenic coupling occurs in infection-induced bone destruction are warranted.
In conclusion, we demonstrate the time course of inflammation resembling osteomyelitis in the TLR2/IL-10-dKO mice. In this model, HIF-1 plays a crucial role in spontaneous bone formation and wound healing. This model will be a valuable tool not only for the further elucidation of the pathobiology of osteomyelitis, but also for the development of new therapies to accelerate bone and wound healing.
Supplementary Material
Highlights.
To date, no animal models of endodontic infection-induced osteomyelitis of the jaws have been reported, limiting our knowledge of the host factors that affect the onset and the course of osteomyelitis. In this study we have characterized the pathogenesis of endodontic infection-induced inflammation resembling osteomyelitis in Toll-like receptor2/interleukin-10 double deficient (TLR2/IL-10-dKO) mice. TLR2/IL-10-dKO mice exhibited rapid progression of osteolytic acute inflammation within 10 days post-infection. However, TLR2/IL-10-dKO mice surprisingly underwent spontaneous resolution of inflammation with reparative bone formation by day 21. We identified that hypoxia-inducible factor 1 alpha subunit and arginase-1 appear to be crucial factors in spontaneous wound healing.
Acknowledgements
The authors thank Drs. Yoshimitsu Abiko (Nihon University School of Dentistry at Matsudo, Japan), Kiichi Hirota (Kansai Medical University, Japan) and Akio Ohta (The Institute of Biomedical Research and Innovation, Japan) for their advice on the experimental design and for helpful discussions. This study was supported in part by NIDCR/NCRR/NIH under awards R21DE023178, R01DE024796 and S10RR027553 to HS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Ishikawa G, Waldron CA. Color Atlas of Oral Pathology. Maryland Heights: Ishiyaku Euroamerica; 1987:104–106. [Google Scholar]
- 2.Asensi V, Alvarez V, Valle E, et al. IL-1 alpha (−889) promoter polymorphism is a risk factor for osteomyelitis. Am J Med Genet A 2003;119A(2):132–136. [DOI] [PubMed] [Google Scholar]
- 3.Herlin T, Fiirgaard B, Bjerre M, et al. Efficacy of anti-IL-1 treatment in Majeed syndrome. Ann Rheum Dis 2013;72(3):410–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofmann SR, Schwarz T, Moller JC, et al. Chronic non-bacterial osteomyelitis is associated with impaired Sp1 signaling, reduced IL10 promoter phosphorylation, and reduced myeloid IL-10 expression. Clin Immunol 2011;141(3):317–327. [DOI] [PubMed] [Google Scholar]
- 5.Takeuchi O, Hoshino K, Akira S. Cutting edge: TLR2-deficient and MyD88-deficient mice are highly susceptible to Staphylococcus aureus infection. J Immunol 2000;165(10):5392–6. [DOI] [PubMed] [Google Scholar]
- 6.Izawa A, Ishihara Y, Mizutani H, et al. Inflammatory bone loss in experimental periodontitis induced by Aggregatibacter actinomycetemcomitans in interleukin-1 receptor antagonist knockout mice. Infect Immun 2014;82(5):1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sasaki H, Hou L, Belani A, et al. IL-10, but not IL-4, suppresses infection-stimulated bone resorption in vivo. J Immunol 2000;165(7):3626–3630. [DOI] [PubMed] [Google Scholar]
- 8.Rider D, Furusho H, Xu S, et al. Elevated CD14 (Cluster of Differentiation 14) and Toll-Like Receptor (TLR) 4 Signaling Deteriorate Periapical Inflammation in TLR2 Deficient Mice. Anat Rec (Hoboken) 2016;299(9):1281–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.AlShwaimi E, Berggreen E, Furusho H, et al. IL-17 receptor A signaling is protective in infection-stimulated periapical bone destruction. J Immunol 2013;191(4):1785–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pautke C, Vogt S, Tischer T, et al. Polychrome labeling of bone with seven different fluorochromes: enhancing fluorochrome discrimination by spectral image analysis. Bone 2005;37(4):441–445. [DOI] [PubMed] [Google Scholar]
- 11.Kong J, Kong F, Gao J, et al. YC-1 enhances the anti-tumor activity of sorafenib through inhibition of signal transducer and activator of transcription 3 (STAT3) in hepatocellular carcinoma. Mol Cancer 2014;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirai K, Furusho H, Hirota K, et al. Activation of hypoxia-inducible factor 1 attenuates periapical inflammation and bone loss. Int J Oral Sci 2018;10(2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan C, Gilbert SR, Wang Y, et al. Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proc Natl Acad Sci U S A 2008;105(2):686–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirota K Involvement of hypoxia-inducible factors in the dysregulation of oxygen homeostasis in sepsis. Cardiovasc Hematol Disord Drug Targets 2015;15(1):29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tagger M, Massler M. Periapical tissue reactions after pulp exposure in rat molars. Oral Surg Oral Med Oral Pathol. 1975;39(2):304–17. [DOI] [PubMed] [Google Scholar]
- 16.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol 2006;59(1):15–26. [DOI] [PubMed] [Google Scholar]
- 17.Iwata T, Kantarci A, Yagi M, et al. Ceruloplasmin induces polymorphonuclear leukocyte priming in localized aggressive periodontitis. J Periodontol 2009;80(8):1300–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stashenko P, Wang CY, Tani-Ishii N, et al. Pathogenesis of induced rat periapical lesions. Oral Surg Oral Med Oral Pathol 1994;78(4):494–502. [DOI] [PubMed] [Google Scholar]
- 19.Takeda N, O’Dea EL, Doedens A, et al. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev 2010;24(5):491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 2012;122(3):787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris AJ, Thompson AR, Whyte MK, et al. HIF-mediated innate immune responses: cell signaling and therapeutic implications. Hypoxia (Auckl) 2014;2:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009;9(3):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riddle RC, Khatri R, Schipani E, et al. Role of hypoxia-inducible factor-1alpha in angiogenic-osteogenic coupling. J Mol Med (Berl) 2009;87(6):583–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 2004;10(8):858–864. [DOI] [PubMed] [Google Scholar]
- 25.Otsuru S, Tamai K, Yamazaki T, et al. Circulating bone marrow-derived osteoblast progenitor cells are recruited to the bone-forming site by the CXCR4/stromal cell-derived factor-1 pathway. Stem Cells 2008;26(1):223–234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.