Abstract
Context
The association between adipsin and glucose metabolism in human subjects remains unclear.
Objective
We investigated the associations between adipsin and insulin resistance/β-cell function in subjects with various degrees of glucose intolerance.
Design
Fasting blood samples were collected for measurements of fasting plasma glucose (FPG), insulin, and adipsin. An oral glucose tolerance test was conducted in subjects with no history of diabetes.
Setting
This study was conducted at a medical center.
Patients
We enrolled 240 subjects with no history of diabetes and 80 patients with known type 2 diabetes (T2D) on diet control or metformin monotherapy.
Main Outcome Measure
β-cell function and insulin resistance were assessed using the homeostasis model assessment (HOMA-β and HOMA-IR, respectively).
Results
Levels of serum adipsin were higher in subjects with normal glucose tolerance (4.0 ± 1.1 µg/mL) or prediabetes (4.0 ± 1.5 µg/mL) compared with subjects with newly diagnosed diabetes (3.8 ± 1.1 µg/mL) or with known T2D on diet control (3.4 ± 1.0 µg/mL) or metformin monotherapy (3.0 ± 1.0 µg/mL, P < 0.001). There was no significant association between adipsin and HOMA-β. In contrast, there was an independent negative association between adipsin and HOMA-IR (β coefficient −0.414, 95% CI −0.720 to −0.109, P = 0.008). The association was more prominent in subjects with a body mass index (BMI) ≥25 kg/m2 or an FPG ≥100 mg/dL (P interaction < 0.001 and 0.014, respectively).
Conclusions
Serum adipsin levels were negatively associated with insulin resistance, especially in subjects with a BMI ≥25 kg/m2 or an FPG ≥100 mg/dL.
Keywords: adipsin, insulin resistance, oral glucose tolerance test
Adipsin is an adipokine that was first described in 1987 [1]. Despite being one of the major proteins secreted by adipocytes [2], a paradoxical decline in adipsin has been noted in animal models of obesity and diabetes [3]. The main function of adipsin is to catalyze the breakdown of complement factor C3 into C3a [4] and activate the complement alternative pathway [5]. Adipsin was later identified as complement factor D [6], which plays an important role in the immune system [7]. Obesity and type 2 diabetes (T2D) are associated with chronic inflammation [8]. Despite an increasing awareness of the interplay between immune system and adipose cell biology [9], the role of adipsin in glucose homeostasis remains unclear.
Activation of the complement pathway has been consistently observed in ob/ob mice and high-fat diet-induced obese mice [10]. In contrast, protection against obesity, reduction in adipose inflammation, and improvement in insulin sensitivity were observed in studies using mice deficient in C3aR1 or treated with an antagonist of the receptor [11, 12]. Lo et al. [13] recently reported that ablation of adipsin in diabetic mice led to insulinopenia and exacerbation of diabetes, whereas restoration of adipsin augmented insulin secretion and improved glucose homeostasis. The authors observed that patients with T2D and β-cell function failure (on insulin therapy) had a lower circulating adipsin level than patients with T2D on metformin therapy, and concluded that adipsin improved β-cell function in diabetes.
Given the aforementioned inconsistent findings, and the limited data in human subjects [14–16] regarding the effects of adipsin on glucose metabolism, more studies are needed to elucidate the mechanism by which adipsin may affect glucose homeostasis. In this study, we aimed to investigate the association between adipsin and insulin resistance/β-cell function in patients with no history of diabetes who underwent an oral glucose tolerance test (OGTT).
1. Subjects and Methods
We enrolled subjects with no history of diabetes who underwent an OGTT to screen for abnormal glucose regulation at our outpatient clinic. Outpatients with known T2D on diet control or metformin monotherapy were enrolled to compare serum adipsin level between patients with known and newly diagnosed abnormal glucose regulation. A fasting blood sample was collected from all study subjects for the measurement of serum adipsin. Written informed consent was provided by all study subjects. This study was approved by the Institutional Review Board of Taichung Veterans General Hospital, Taichung, Taiwan, and was conducted in accordance with the Declaration of Helsinki.
A standard 75-g OGTT [17] was conducted in subjects with no history of diabetes after an overnight fast. A fasting blood sample was collected for the measurement of glycated hemoglobin (HbA1c), insulin, and adipsin. For outpatients with known T2D, levels of serum adipsin were determined using fasting blood samples collected for measurements of fasting plasma glucose (FPG) and HbA1c at their outpatient clinic visit. Relevant clinical parameters, such as body mass index (BMI), systolic and diastolic blood pressure, duration of diabetes, and lipids profiles, were collected from hospital records.
Serum adipsin was determined using an ELISA kit (Quantikine® Human Complement Factor D Immunoassay, Catalog Number DFD00; R&D Systems, Minneapolis, MN) [18] following the manufacturer’s instructions. The mean minimal detectable concentration of adipsin was 0.013 ng/mL, and the intra- and interassay coefficients of variation were <6.4% and <9.0%, respectively. Plasma glucose was measured using the glucose oxidase-peroxidase method (Wako Diagnostics, Tokyo, Japan). The intra- and interassay coefficients of variation for glucose (range, 0 to 800 mg/dL) were both <1.5%. Plasma insulin was determined using an electrochemiluminescence immunoassay (Elecsys 2010; Roche Diagnostics, Indianapolis, IN). The intra- and interassay coefficients of variation for insulin were 1.8% and 2.5%, respectively. HbA1c was measured by boronate-affinity high-performance liquid chromatography (CLC385TM, Primus Corporation, Kansas City, MO). The intra- and interassay coefficients of variation for HbA1c (range, 4.2% to 19.6%) were <0.9% and <2.9%, respectively.
For subjects with no history of diabetes, their glucose regulation status was determined according to the results of OGTT and HbA1c, as recommended by the American Diabetes Association [19]. We assessed insulin resistance and β-cell function using the homeostasis model assessment [20] of insulin resistance and β-cell function (HOMA-IR and HOMA-β, respectively). HOMA-IR = fasting insulin [μU/L] * fasting glucose [mmol/L] / 22.5. HOMA-β = 20 × fasting insulin [μU/L] / (fasting glucose [mmol/L] − 3.5).
The Statistical Package for the Social Sciences (IBM SPSS version 22.0; International Business Machines Corp, NY) was used for all of the statistical analyses. Continuous variables are reported as mean ± SD and categorical data are given as number (percentage). The Student t test or one-way ANOVA (posthoc analysis using the Bonferroni test) was used to test statistically significant differences in continuous variables between groups, whereas the χ2 test was used for categorical variables. A generalized linear model with fixed effect was used to examine the association of adipsin with insulin resistance (HOMA-IR) and β-cell function (HOMA-β). Interactions of clinical parameters on the association between adipsin and HOMA-IR/HOMA-β were also examined using a generalized linear model. In all of the statistical analyses, a two-sided P value of less than 0.05 was considered statistically significant.
2. Results
From July 2013 to January 2015, a total of 320 subjects were enrolled in this study. Table 1 shows the distribution of the study population. Among the 320 subjects included in this study, 240 subjects with no history of diabetes underwent an OGTT and HbA1c test to determine their glucose regulation status (group 1~group 3), whereas 80 subjects with known T2D were on diet control or metformin monotherapy (group 4 and group 5, respectively). Table 2 shows the characteristics of the study subjects according to their glucose regulation status. Subjects with normal glucose tolerance (group 1) had a lower BMI, a lower systolic and diastolic blood pressure, lower levels of triglycerides, FPG, and HbA1c, as well as a higher level of serum adipsin, compared with subjects with prediabetes (group 2), newly diagnosed diabetes (group 3), or known T2D (group 4 and group 5). Among subjects who had undergone an OGTT (group 1~3), 2-hour plasma glucose was lower, whereas HOMA-β was higher in subjects in group 1, compared with subjects in group 2 and group 3. There was no significant between-group difference in HOMA-IR.
Table 1.
Distribution of the Study Population
| All Participants | N = 320 |
|---|---|
| Participants with no history of diabetes | n = 240 |
| Normal glucose tolerance by OGTT and HbA1c (group 1, n = 58) | |
| Prediabetes by OGTT and HbA1c (group 2, n = 140) | |
| Newly diagnosed diabetes by OGTT and HbA1c (group 3, n = 42) | |
| Participants with known T2D | n = 80 |
| On diet control (group 4, n = 40) | |
| On metformin monotherapy (group 5, n = 40) |
Table 2.
Characteristics of the Study Population According to Their Glucose Regulation Status
|
|
Subjects With No History of Diabetes |
Subjects With Known T2D |
|
|||
|---|---|---|---|---|---|---|
| Variable | Group 1 (NGT) | Group 2 (Prediabetes) | Group 3 (New Diabetes) | Group 4 (on Diet Control) | Group 5 (on Metformin) | P |
| N | 58 | 140 | 42 | 40 | 40 | |
| Age, y | 58.6 ± 12.1 | 61.3 ± 11.5 | 63.0 ± 12.1 | 62.3 ± 10.5 | 63.7 ± 10.1 | 0.180 |
| Male, n (%) | 43 (74.1) | 117 (83.6) | 36 (85.7) | 34 (85.0) | 34 (85.0) | 0.468 |
| BMI, kg/m2 | 24.9 ± 3.1 | 26.1 ± 3.7 | 27.1 ± 3.7 | 26.9 ± 3.5 | 26.6 ± 3.4 | 0.017 |
| Systolic BP, mm Hg | 122 ± 19 | 128 ± 18 | 129 ± 19 | 131 ± 19 | 133 ± 11 | 0.047 |
| Diastolic BP, mm Hg | 73 ± 10 | 75 ± 11 | 74 ± 10 | 80 ± 14 | 79 ± 9 | 0.007 |
| Smoking, n (%) | 7 (12.1) | 12 (8.6) | 6 (14.3) | 5 (12.5) | 7 (17.5) | 0.565 |
| Diabetes duration, y | N/A | N/A | N/A | 4.4 ± 5.3 | 4.8 ± 6.8 | <0.001 |
| Total cholesterol, mg/dL | 177 ± 50 | 168 ± 33 | 165 ± 40 | 184 ± 42 | 175 ± 36 | 0.154 |
| LDL cholesterol, mg/dL | 115 ± 45 | 106 ± 30 | 100 ± 28 | 114 ± 38 | 110 ± 34 | 0.187 |
| HDL cholesterol, mg/dL | 48 ± 14 | 47 ± 14 | 43 ± 11 | 47 ± 12 | 46 ± 10 | 0.289 |
| Triglycerides, mg/dL | 128 ± 72 | 132 ± 74 | 171 ± 156 | 203 ± 176 | 147 ± 63 | 0.002 |
| Fasting PG, mg/dL | 88 ± 6 | 94 ± 7 | 108 ± 19 | 132 ± 46 | 139 ± 33 | <0.001 |
| OGTT 2-h PG, mg/dL | 112 ± 21 | 144 ± 31 | 229 ± 45 | N/A | N/A | <0.001 |
| HbA1c, % | 5.3 ± 0.2 | 5.7 ± 0.4 | 6.4 ± 0.7 | 7.0 ± 1.4 | 7.3 ± 1.4 | <0.001 |
| HOMA-IRa | 2.3 ± 1.2 | 2.6 ± 2.0 | 3.0 ± 1.9 | N/A | N/A | 0.129 |
| HOMA-βa | 152 ± 82 | 128 ± 93 | 121 ± 110 | N/A | N/A | 0.009 |
| Adipsin, µg/mLa | 4.0 ± 1.1 | 4.0 ± 1.5 | 3.8 ± 1.1 | 3.4 ± 1.0 | 3.0 ± 1.0 | <0.001 |
Values are mean ± SD or n (%).
Abbreviations: BP, blood pressure; HDL, high-density lipoprotein; HOMA, homeostasis model assessment; IR, insulin resistance; LDL, low-density lipoprotein; N/A, not applicable; NGT, normal glucose tolerance; PG, plasma glucose.
Log transformed (base 10) before analysis.
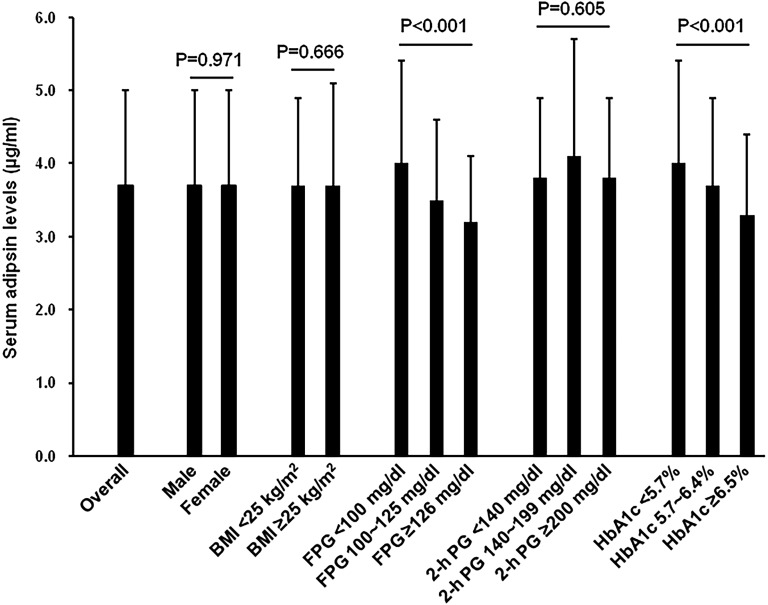
Figure 1 shows levels of serum adipsin in subgroups of the study population by gender, BMI, FPG, 2-hour plasma glucose, and HbA1c. Overall, the mean adipsin level was 3.7 μg/mL. There was no significant between-group difference in serum adipsin levels when study subjects were divided into two groups by gender (male vs female) or BMI (<25 vs ≥25 kg/m2). When we grouped study subjects according to their FPG, 2-hour plasma glucose, and HbA1c levels, we observed that mean serum adipsin level was higher in subjects with a FPG <100 mg/dL or an HbA1c <5.7%, compared with those who had a FPG ≥100 m/dL or an HbA1c ≥5.7%. However, there was no significant between-group difference in serum adipsin level when study subjects were grouped according to their 2-hour plasma glucose (<140 vs ≥140 mg/dL).
Figure 1.
Levels of serum adipsina in subgroups of the study population. Error bars represent one SD. 2-h PG, OGTT 2-h plasma glucose. aLog transformed (base 10) before analysis.
We examined the associations of serum adipsin level with HOMA-β and HOMA-IR. There was no significant association between adipsin and HOMA-β (β coefficient, −0.320; 95% CI, −0.645 to 0.006; P = 0.054). In contrast, there was a significant negative association between adipsin and HOMA-IR (β coefficient, −0.449; 95% CI, −0.762 to −0.135; P = 0.005, Table 3). The negative association between adipsin and HOMA-IR remained significant after adjustment for confounders (β coefficient, −0.414; 95% CI, −0.720 to −0.109; P = 0.008; Table 3), such as age, gender, and BMI.
Table 3.
Generalized Linear Model With HOMA-IRa as the Dependent Variable
| Independent Variable | β Coefficient | 95% CI | P |
|---|---|---|---|
| Adipsin (μg/mL)a | |||
| Model 1 | −0.449 | −0.762, −0.135 | 0.005 |
| Model 2 | −0.477 | −0.773, −0.180 | 0.002 |
| Model 3 | −0.471 | −0.777, −0.165 | 0.003 |
| Model 4 | −0.414 | −0.720, −0.109 | 0.008 |
Model 1, unadjusted; Model 2, adjusted for age, gender, and BMI; Model 3, adjusted for variables in Model 2 plus systolic blood pressure, smoking, total cholesterol, and triglycerides; Model 4, adjusted for variables in Model 3 plus FPG and HbA1c.
Abbreviations: HOMA, homeostasis model assessment. IR, insulin resistance.
Log transformed (base 10).
We examined the effects of gender, BMI, FPG, 2-hour plasma glucose, and HbA1c on the negative association between adipsin and HOMA-IR. As shown in Table 4, we found that gender, 2-hour plasma glucose, and HbA1c had no significant effect on the negative association between adipsin and HOMA-IR (P interaction > 0.05). In contrast, both BMI and FPG had a significant effect (P interaction < 0.001 and 0.014, respectively). A negative association between adipsin and HOMA-IR was observed in subjects with a BMI ≥25 kg/m2 or a FPG ≥100 mg/dL.
Table 4.
| Independent Variable | β Coefficient | 95% CI |
P Interaction | |
|---|---|---|---|---|
| Adipsin (μg/mL)b | −0.414 | −0.720, −0.109 | ||
| Subgroups | ||||
| Gender | 0.307 | |||
| Male | −0.563 | −0.901, −0.225 | ||
| Female | 0.134 | −0.667, 0.936 | ||
| BMI | <0.001 | |||
| <25.0 kg/m2 | 0.318 | −0.143, 0.778 | ||
| ≥25.0 kg/m2 | −0.840 | −1.273, −0.406 | ||
| FPG | 0.014 | |||
| <100 mg/dL | −0.244 | −0.599, 0.112 | ||
| ≥100 mg/dL | −0.704 | −1.332, −0.076 | ||
| 2-h plasma glucose | 0.122 | |||
| <140 mg/dL | −0.427 | −0.839, −0.014 | ||
| ≥140 mg/dL | −0.441 | −0.908, 0.027 | ||
| HbA1c | 0.338 | |||
| <5.7% | −0.144 | −0.566, 0.278 | ||
| ≥5.7% | −0.789 | −1.249, −0.329 | ||
Abbreviations: HOMA, homeostasis model assessment; IR, insulin resistance.
Adjusted for age, gender, BMI, systolic blood pressure, smoking, total cholesterol, triglycerides, FPG, and HbA1c.
Log transformed (base 10).
3. Discussion
In this study, we demonstrated that serum adipsin was negatively associated with insulin resistance (HOMA-IR), especially in subjects with a BMI ≥25 kg/m2 or a FPG ≥100 mg/dL. Lo et al. [13] recently reported that adipsin improved β-cell function in diabetic mice, and a lower circulating adipsin level was observed in patients with T2D and β-cell function failure (on insulin therapy), compared with those with T2D on metformin therapy. However, they did not report an association between circulating adipsin and β-cell function/insulin resistance in human subjects. In this study, we observed that serum adipsin level was highest in subjects with normal glucose tolerance, and was lower in subjects with newly diagnosed diabetes, with known T2D patients having the lowest level (Table 2). Nevertheless, we found that adipsin was not associated with β-cell function (HOMA-β). In contrast, adipsin was negatively associated with insulin resistance (HOMA-IR), independent of several confounders (Table 3).
Consistent with our results, Zhou et al. [16] recently reported that levels of serum adipsin were lower in Chinese subjects with newly diagnosed T2D and impaired glucose tolerance, compared with those with normal glucose tolerance. Although the total number of patients in their study was relatively small (n = 137), the authors observed a positive correlation between adipsin and HOMA-β, whereas a negative correlation between adipsin and HOMA-IR. Their finding of the association between circulating adipsin levels and insulin resistance was not consistent with previous reports. In a study conducted in 74 pregnant women [14], the authors reported a positive correlation between fetal adipsin concentrations and fetal/maternal HOMA-IR. In contrast, another study conducted in 379 adults [15] reported no significant association between circulating adipsin and HOMA-IR. The inconsistent findings might be explained in part by the different study populations. Our study has several strengths. First, we recruited a relatively large number of study subjects. Second, we enrolled subjects with no history of diabetes and subjects with known T2D for comparison. Third, we conducted an OGTT in subjects with no history of diabetes to confirm their glucose regulation status. The negative association between adipsin and HOMA-IR in subjects with no history of diabetes suggests that adipsin may be involved in the pathogenesis of abnormal glucose metabolism.
The mechanism by which adipsin was negatively associated with HOMA-IR might be related to inflammation. T2D is a chronic inflammatory disease [21, 22], whereas insulin resistance is a key factor that leads to deterioration of glucose homeostasis in the early course of the disease [23, 24]. It has been reported that inflammatory cytokines are elevated in patients with T2D. For example, IL-17, which was reported to suppress differentiation of preadipocytes and impair adipsin expression in adipocytes [25], was elevated in patients with T2D [26, 27]. Furthermore, IL-17 has been associated with insulin resistance in animal models [28]. The aforementioned results in previous studies may help to explain our findings that showed decreased adipsin in subjects with newly diagnosed diabetes and known T2D (Table 2), and there was an independent negative association between adipsin and HOMA-IR (Table 3). Interestingly, the negative association between adipsin and HOMA-IR was observed in subjects with a BMI ≥25 kg/m2 or a FPG ≥100 mg/dL (Table 4). This finding may not be surprising, as both obesity and FPG are known to be associated with insulin resistance [29, 30] in human subjects.
There are several limitations in this study. First, the cross-sectional study design did not allow us to draw conclusions about adipsin in the pathogenesis of T2D. This issue needs to be addressed in prospective studies. Second, we did not measure inflammatory markers or cytokines. Thus, the association between inflammation, adipsin, and insulin resistance could not be investigated in this study. Finally, we enrolled subjects with no history of diabetes who underwent an OGTT to screen for abnormal glucose regulation. Our results may not be generalized to other populations, such as patients with long-standing diabetes or chronic diabetes complications, and therefore further studies are needed.
In summary, we demonstrated that serum adipsin was negatively associated with insulin resistance (HOMA-IR), especially in subjects with a BMI ≥25 kg/m2 or a FPG ≥100 mg/dL. Our findings suggest that adipsin may be involved in the pathogenesis of abnormal glucose metabolism, and therefore further investigation is warranted.
Acknowledgments
The authors are grateful to the study subjects for their participation.
Financial Support: This work was supported by The National Science Council, Taiwan [NSC MOST 104-2314-B-075A-003; MOST 107-2314-B-075A-001-MY3]; and Taichung Veterans General Hospital, Taichung, Taiwan [TCVGH-1043501B, TCVGH-1070101C, TCVGH-1070102D].
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BMI
body mass index
- FPG
fasting plasma glucose
- HbA1c
glycated hemoglobin
- OGTT
oral glucose tolerance test
- T2D
type 2 diabetes
References and Notes
- 1. Cook KS, Min HY, Johnson D, Chaplinsky RJ, Flier JS, Hunt CR, Spiegelman BM. Adipsin: a circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science. 1987;237(4813):402–405. [DOI] [PubMed] [Google Scholar]
- 2. White RT, Damm D, Hancock N, Rosen BS, Lowell BB, Usher P, Flier JS, Spiegelman BM. Human adipsin is identical to complement factor D and is expressed at high levels in adipose tissue. J Biol Chem. 1992;267(13):9210–9213. [PubMed] [Google Scholar]
- 3. Flier JS, Cook KS, Usher P, Spiegelman BM. Severely impaired adipsin expression in genetic and acquired obesity. Science. 1987;237(4813):405–408. [DOI] [PubMed] [Google Scholar]
- 4. Cianflone K, Roncari DA, Maslowska M, Baldo A, Forden J, Sniderman AD. Adipsin/acylation stimulating protein system in human adipocytes: regulation of triacylglycerol synthesis. Biochemistry. 1994;33(32):9489–9495. [DOI] [PubMed] [Google Scholar]
- 5. Xu Y, Ma M, Ippolito GC, Schroeder HW Jr, Carroll MC, Volanakis JE. Complement activation in factor D-deficient mice. Proc Natl Acad Sci USA. 2001;98(25):14577–14582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rosen BS, Cook KS, Yaglom J, Groves DL, Volanakis JE, Damm D, White T, Spiegelman BM. Adipsin and complement factor D activity: an immune-related defect in obesity. Science. 1989;244(4911):1483–1487. [DOI] [PubMed] [Google Scholar]
- 7. Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11(9):785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. [DOI] [PubMed] [Google Scholar]
- 9. Shu CJ, Benoist C, Mathis D. The immune system’s involvement in obesity-driven type 2 diabetes. Semin Immunol. 2012;24(6):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang J, Wright W, Bernlohr DA, Cushman SW, Chen X. Alterations of the classic pathway of complement in adipose tissue of obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2007;292(5):E1433–E1440. [DOI] [PubMed] [Google Scholar]
- 11. Mamane Y, Chung Chan C, Lavallee G, Morin N, Xu LJ, Huang J, Gordon R, Thomas W, Lamb J, Schadt EE, Kennedy BP, Mancini JA. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes. 2009;58(9):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim J, Iyer A, Suen JY, Seow V, Reid RC, Brown L, Fairlie DP. C5aR and C3aR antagonists each inhibit diet-induced obesity, metabolic dysfunction, and adipocyte and macrophage signaling. FASEB J. 2013;27(2):822–831. [DOI] [PubMed] [Google Scholar]
- 13. Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, Kelly ME, Chatterjee Bhowmick D, Murano I, Cohen P, Banks AS, Khandekar MJ, Dietrich A, Flier JS, Cinti S, Blüher M, Danial NN, Berggren PO, Spiegelman BM. Adipsin is an adipokine that improves β cell function in diabetes. Cell. 2014;158(1):41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sivakumar K, Bari MF, Adaikalakoteswari A, Guller S, Weickert MO, Randeva HS, Grammatopoulos DK, Bastie CC, Vatish M. Elevated fetal adipsin/acylation-stimulating protein (ASP) in obese pregnancy: novel placental secretion via Hofbauer cells. J Clin Endocrinol Metab. 2013;98(10):4113–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abu-Farha M, Behbehani K, Elkum N. Comprehensive analysis of circulating adipokines and hsCRP association with cardiovascular disease risk factors and metabolic syndrome in Arabs. Cardiovasc Diabetol. 2014;13(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou Q, Ge Q, Ding Y, Qu H, Wei H, Wu R, Yao L, Wei Q, Feng Z, Long J, Deng H. Relationship between serum adipsin and the first phase of glucose-stimulated insulin secretion in individuals with different glucose tolerance. J Diabetes Investig. 2018;9(5):1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Report of a WHO consultation, 1999. Available at: http://whqlibdoc.who.int/hq/1999/WHO_NCD_NCS_99.2.pdf. Accessed 18 September 2018.
- 18.RRID. AB_2754975.
- 19. American Diabetes Association Standards of medical care in diabetes. Diabetes Care. 2018;41(Suppl 1):S13–S27. [DOI] [PubMed] [Google Scholar]
- 20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 21. Lazar MA. How obesity causes diabetes: not a tall tale. Science. 2005;307(5708):373–375. [DOI] [PubMed] [Google Scholar]
- 22. Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38(3):183–191. [DOI] [PubMed] [Google Scholar]
- 23. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988;37(12):1595–1607. [DOI] [PubMed] [Google Scholar]
- 24. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014;383(9922):1068–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zúñiga LA, Shen WJ, Joyce-Shaikh B, Pyatnova EA, Richards AG, Thom C, Andrade SM, Cua DJ, Kraemer FB, Butcher EC. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J Immunol. 2010;185(11):6947–6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kologrivova IV, Suslova TE, Koshel’skaya OA, Vinnitskaya IV, Trubacheva OA. System of matrix metalloproteinases and cytokine secretion in type 2 diabetes mellitus and impaired carbohydrate tolerance associated with arterial hypertension. Bull Exp Biol Med. 2014;156(5):635–638. [DOI] [PubMed] [Google Scholar]
- 27. Liu S, Lin YU, Liu X. Protective effects of SIRT1 in patients with proliferative diabetic retinopathy via the inhibition of IL-17 expression. Exp Ther Med. 2016;11(1):257–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chuang HC, Sheu WH, Lin YT, Tsai CY, Yang CY, Cheng YJ, Huang PY, Li JP, Chiu LL, Wang X, Xie M, Schneider MD, Tan TH. HGK/MAP4K4 deficiency induces TRAF2 stabilization and Th17 differentiation leading to insulin resistance. Nat Commun. 2014;5(1):4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Czech MP. Insulin action and resistance in obesity and type 2 diabetes. Nat Med. 2017;23(7):804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T; Impaired Glucose Tolerance for Atherosclerosis and Diabetes study . Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care. 2003;26(3):868–874. [DOI] [PubMed] [Google Scholar]