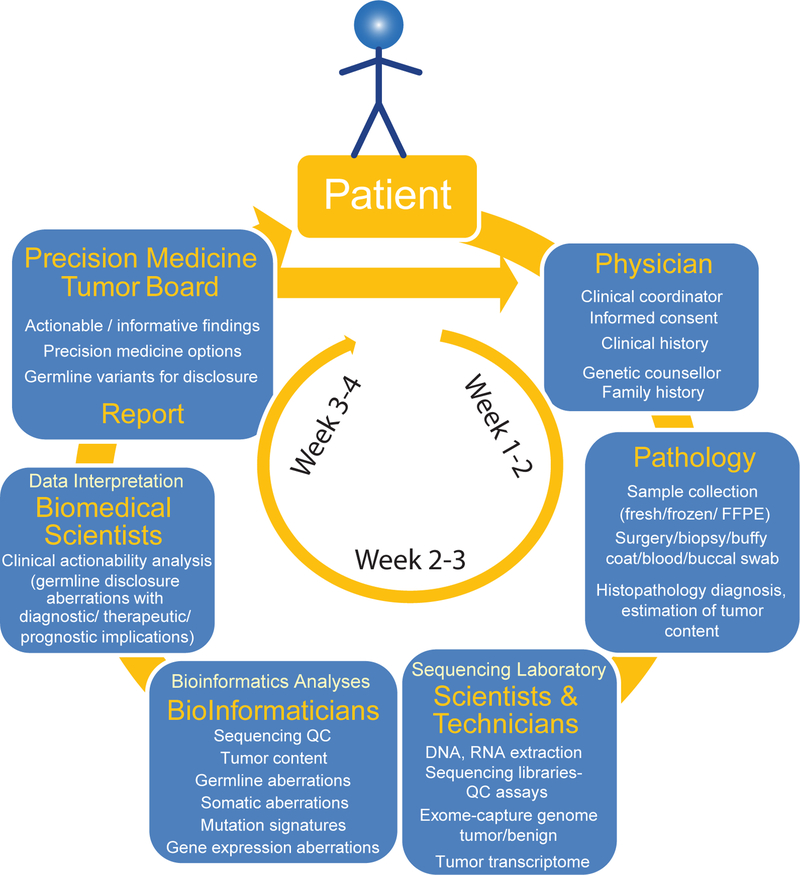
Figure 3. Workflow of integrative clinical sequencing for precision oncology.
The patient, in consultation with the cancer physician, enters the clinical sequencing program upon signing the informed consent. Along with documentation of detailed clinical history, a genetic counselor obtains family history of the patient to assess likely hereditary predisposition to cancer. Patient’s tumor biopsy is flash frozen in OCT blocks, and, along with blood or buccal swab, the samples are sent to the CLIA-certified sequencing laboratory. Histology sections of the tumor biopsy blocks are evaluated by a clinical pathologist for diagnosis and tumor content. DNA and RNA from tissue blocks with the highest tumor content and DNA from blood/buccal samples are used to generate sequencing libraries. Exome capture libraries from germline and tumor samples and the transcriptome library from tumor RNA are analyzed for germline and somatic aberrations. Potentially actionable molecular observations are identified and discussed at the multidisciplinary precision medicine tumor board (see also BOX 1), and a summary report of clinical recommendations is provided to the attending physician.