Figure 3.
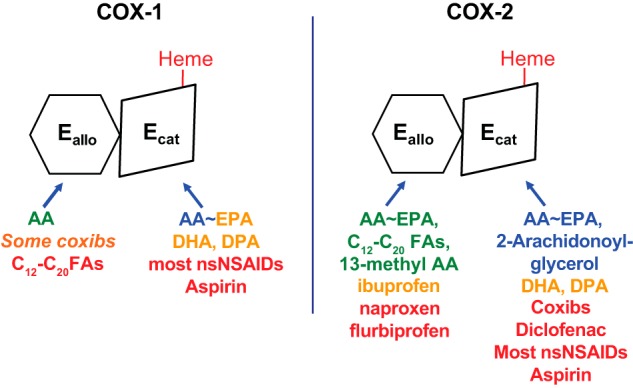
Isoform-specific interactions of COX substrates, nonsubstrate FAs, and COX inhibitors with huCOX-1 and huCOX-2. Each COX isoform functions as a conformational heterodimer composed of an allosteric (Eallo) and a catalytic (Ecat) subunit. The individual subunits of human COXs differ both in their affinities for different ligands and in their responses to binding of the ligands. Efficient COX substrates are shown in blue in the approximate order of their catalytic efficiencies. FAs that are inefficient COX substrates can interfere with prostaglandin formation, typically by competing with AA for the Ecat; these are shown in orange (e.g. EPA, DHA, and DPA). Ligands shown in green allosterically stimulate COX activity. Ligands shown in red interfere with COX activity either allosterically by binding Eallo or competitively by binding Ecat. Ligands that bind Eallo can also affect responses to COX inhibitors. For example, nonsubstrate FAs bound to Eallo of huCOX-1 increase the rate of aspirin acetylation, whereas celecoxib (in orange) bound to Eallo of huCOX-1 can interfere with aspirin action. This figure and the legend are adapted from Ref. 64.
