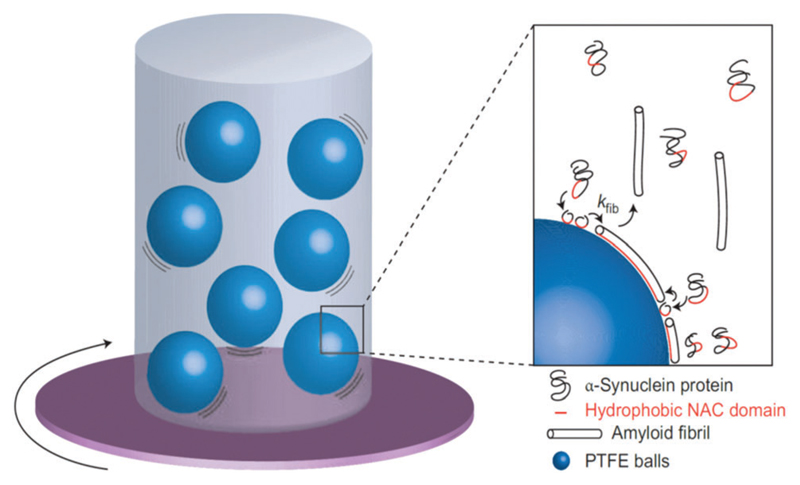
Fig. 4.
Formation of amyloid fibrils by α-synuclein. A solution was agitated in the presence of particles of hydrophobic PTFE, slightly hydrophilic PMMA or chemically inert glass; experiments with air injected at the top of the cuvette were also performed. Inset: Proposed mechanism of the fibrillization. The aggregation of proteins into fibrils (at the rate constant kfib), caused by association of the protein hydrophobic NAC domains, is enhanced in the presence of the hydrophobic PTFE interface. Reprinted with permission from ref. 158. Copyright 2010, Nature Publishing Group.