Figure 2.
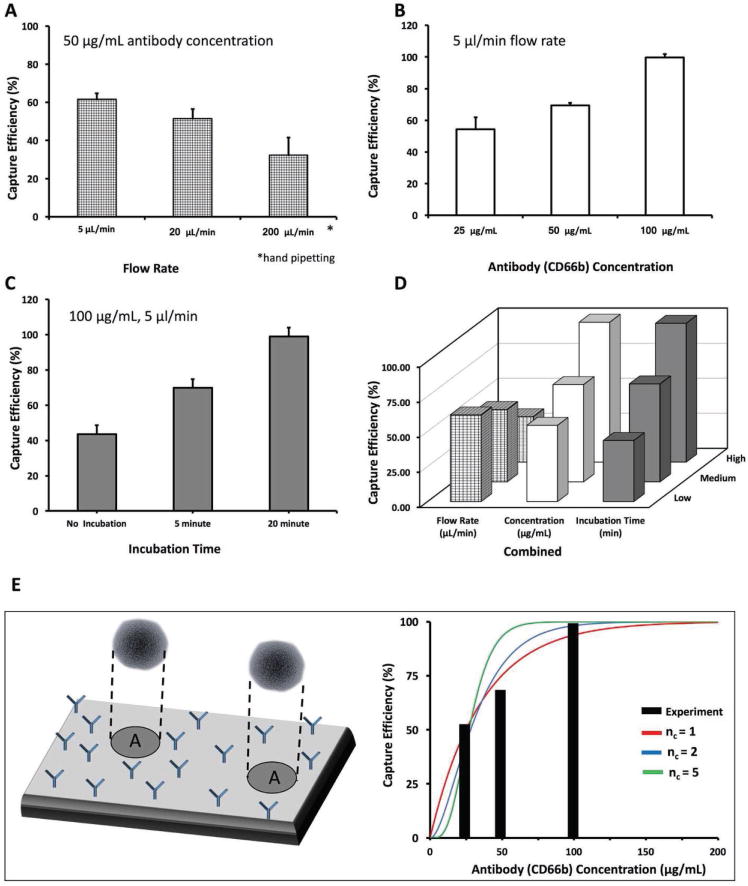
Optimization of the experimental variables. To define the optimum conditions for cell capture, we examined the effect of three interrelated variables: flow rate, capture antibody concentration, and incubation time of blood. A) To optimize the flow rate, we kept the antibody concentration constant and varied the flow rate. We observed that the highest capture efficiency was achieved with 5 μL min−1 flow rate. B) For optimizing antibody concentration, we kept the flow rate constant at 5 μL min−1, and varied the antibody concentration. We observed that the highest capture efficiency was achieved using 100 μg mL−1. C) For optimizing the incubation time, we kept the antibody concentration at 100 μg mL−1, the flow rate at 5 μL min−1, and used three different time periods. D) A graph of all these three parameters together, showing that the highest capture efficiency was achieved with 20 min incubation time, 5 μL min−1 flow rate, and 100 μg mL−1 antibody concentration. E) Results of a theoretical model that uses a Poisson distribution of cells within the microfluidic channel surface, captured by varying number of surface antibodies (anti-CD66b antibodies). The model suggests that the 2–5 binding sites per neutrophil is enough to achieve the observed collection efficiencies and fits the form of the experimental efficiency curve.
