Figure 4. Structure of ZmALDH12.
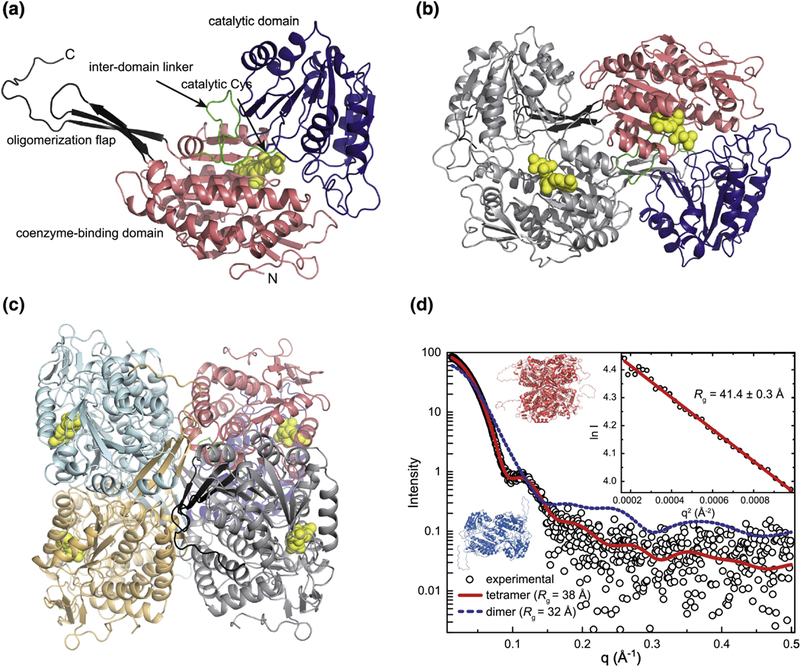
(a) A subunit of ZmALDH12 highlighting domain architecture. (b) The domain-swapped dimer. One subunit is colored according to domains as in the first panel. The other subunit has a single color. The coenzyme molecule is in yellow. (c) The ZmALDH12 tetramer formed in solution. One subunit is colored according to domains as in panel a. The other subunits have single colors. (d) An experimental SAXS curve for ZmALDH12 (open circles) extrapolated to infinite dilution. The inset shows the Guinier plot. The red curve was calculated from a complete model generated by AllosMod-FoXS using the crystallographic dimer-of-dimers tetramer as a template (χ2 = 0.6). The blue dashed curve was calculated from a ZmALDH12 crystallographic dimer (Figure 4b).
