Abstract
Objective:
To investigate the impact of corticosteroid therapy on the growth of participants in the Steroids in Biliary Atresia Randomized Trial (START) conducted through the Childhood Liver Disease Research Network. The primary analysis in START indicated that steroids did not have a beneficial effect on drainage in a cohort of infants with biliary atresia (BA). We hypothesized that steroids would have a detrimental effect on growth in these infants.
Study design:
140 infants were enrolled in START, with 70 randomized to each treatment arm: steroid and placebo. Length, weight, and head circumference were obtained at baseline and follow up visits to 24 months of age.
Results:
Steroid treated patients had significantly lower length and head circumference z-scores during the first 3 months post Hepatoportoenterostomy (HPE), and significantly lower weight until 12 months. Growth trajectories in the steroid and placebo arms differed significantly for length (p <0.0001), weight (p=0.009), and head circumference (p<0.0001) with the largest impact noted for those with successful HPE. Growth trajectory for head circumference was significantly lower in steroid treated patients irrespective of HPE status, but recovered during the second 6 months of life.
Conclusions:
Steroid therapy following HPE in BA is associated with impaired length, weight, and head circumference growth trajectories for at least 6 months post-HPE, especially impacting infants with successful bile drainage.
Keywords: Chronic Liver Disease, Sarcopenia, Failure to Thrive
Biliary atresia (BA) is the most common cause of cirrhosis with end stage liver disease in infancy and is the leading indication for liver transplantation (LT) in early childhood. Hepatoportoenterostomy (HPE) is successful in extending transplant-free survival in approximately 50% of patients, delaying the need for transplantation into later childhood or beyond (1–3).
There have been multiple focused efforts to improve transplant-free survival by refining the surgical procedure and maximizing medical management over the past thirty years (4). One of the most recent was the Steroids in Biliary Atresia Randomized Trial (START) conducted through the Childhood Liver Disease Research Network (ChiLDReN) (5). This randomized, multi-center, double-blind, placebo controlled trial of steroid therapy following HPE in 140 infants with BA did not demonstrate a treatment benefit in bile drainage at 6 months or survival with native liver at 24 months (5). Furthermore, steroid treatment was associated with earlier onset of serious adverse events. The trial included standardized measurement of growth in participating infants and represents one of the largest and most comprehensive anthropometric data sets in a population of infants with chronic and end stage liver disease.
Growth failure and malnutrition are common in children with advanced liver disease and have been attributed to multiple factors. These include increased energy requirements, derangements in metabolism, anorexia, early satiety, abnormal nutrient absorption, and alterations in the growth hormone axis. (6–10) Growth failure and malnutrition increase the risk of complications and mortality in infants requiring liver transplant. (11, 12) Although children display accelerated growth following successful transplant, catch up growth is frequently incomplete in infants with severe deficits prior to transplant. (13, 14)
In a retrospective study of 100 infants with BA, our group previously reported that deficits in weight and length were most pronounced in infants with unsuccessful HPE and that slower growth was an early indicator of poor outcome in those with incomplete drainage. (15) The focus of this report is to detail growth measurements in the patients included in the START trial and examine the impact of steroid exposure on growth trajectory in this vulnerable infant group. This analysis expands upon our previous work by including head circumference, mid-arm circumference, and triceps skin fold, which can improve accuracy in assessment of nutritional status in infants with end stage liver disease in whom ascites and organomegaly confound weight measurement. Differences in growth trajectory are compared by HPE outcome to further our understanding of the impact of progressive cholestatic liver disease in infancy.
METHODS
Study Design
The START protocol, methods, and baseline characteristics of the cohort have been described previously (5). Between September 2005 and February 2011, 140 infants with BA were randomly assigned to receive double-blind therapy with either steroid or placebo. Fourteen clinical sites in the ChiLDReN network participated in the study. Randomization was performed centrally by the data coordinating center and was stratified by study center in blocks of four. Treated patients received methylprednisolone or prednisolone at a dose of 4 mg/kg/d (weeks 1–2), 2 mg/kg/day (weeks 3–4), 1 mg/kg/day (weeks 5–6) and were subsequently tapered off over a 7 week period. Of note, participants received medium chain triglyceride enriched formula in quantities adequate to ensure appropriate caloric intake. However, recording of actual volume of formula ingested or calorie counts were not part of the study protocol.
Patient Population
Infants who had BA and had been enrolled in the ChiLDReN PRospective OBsErvational study of cholestasis in infancy (PROBE) and later underwent HPE were recruited. Inclusion criteria were age 180 days or younger, serum direct or conjugated bilirubin level of 2 mg/dL and higher, and greater than 20% of total bilirubin, post-conception age of 36 weeks or older, and weight of 2000 g or greater. Exclusion criteria included previous hepatobiliary surgery, known immunodeficiency, diabetes mellitus, or significant systemic hypertension for age.
Growth Measures
Length , weight, head circumference, mid-arm circumference, and triceps skin fold thickness were obtained at baseline and at 1, 2, 3, and 6 months following HPE, and measurements were repeated at 12, 18, and 24 months of chronologic age. Weight was also measured at 2 weeks following HPE. All measurements were performed according to standardized practices and patients were placed in the recumbent position for length measurements. Z-scores were calculated using SAS macros provided by the Centers for Disease Control and Prevention (CDC) [https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/index.htm]. Length, weight, weight to length and head circumference z-scores were calculated using the CDC growth charts. Arm circumference and skinfold thickness z-scores were calculated using the World Health Organization (WHO) growth charts, using the maximum age of 730 days allowable by the macro for participants 731–737 days old at their last visit (n=4 arm circumference measures, n=3 skinfold thickness measures). Arm measures for the unsuccessful HPE group at 18 months and 2 years are not included in the analysis due to small numbers. Additionally, arm measures in the successful HPE group from >737 days of age were excluded from analysis (n=19). Z-scores flagged by the programs as not biologically plausible were excluded from analysis (up to 2% of values). Additionally, length z-scores and head circumference with a change of >=1 between visits were excluded from the longitudinal analysis (up to 1% of values).
Statistical Analysis
Descriptive statistics, mean and standard deviation (SD) of all outcome measures at all time points were calculated by treatment arms. At each time point, we compared these outcomes to the normal population using a Kolmogorov-Smirnov goodness-of-fit test compared to the standard normal distribution. Comparisons between treatment arms were made using a Kolmogorov-Smirnov two-sample test. P-values were not adjusted for multiple comparisons. Successful HPE was defined as a total bilirubin < 1.5 mg/dl at 6 months post-HPE with native liver, unsuccessful otherwise. Because HPE outcome has significant effect on growth trajectory (15), we also calculated descriptive statistics for all outcome measures stratified by HPE outcome.
Given that 40% of participants either died (n=5) or were transplanted (n=50) prior to the 24 month time point, a joint model of the repeated growth measure and transplant or death time was used to compare the longitudinal growth trajectory between the two arms. (16) This analysis can adjust for the potential bias due to informative dropout caused by transplant and death. Penalized B-splines were used in the model to capture the non-linear growth trajectories (17). χ2 test was used to compare whether the overall growth trajectories differ between placebo and steroid groups. Estimated mean trajectories and their 95% confidence intervals were plotted to visually present the difference between the two groups and help interpret the testing results. The comparison was performed for the overall study population and separately for participants with successful versus unsuccessful HPE.
RESULTS
The trial included 140 patients, with 70 randomized to each arm, 51% female, 64% white, and 4% with BA splenic malformation (Table 3 online only). Enrollment began in September 2005 and ended in February 2011. Mean age at HPE was 2.3±0.9 months. As previously described (5), the primary study end-point of serum bilirubin level less than 1.5 mg/dL with native liver 6 months following HPE was met in 58.6% of steroid treated patients versus 48.6% of placebo treated patients; adjusted relative risk (RR) 1.14 [95% CI, 0.83–1.57, p=0.43]. Overall survival with native liver at 24 months was 59% (58.7% steroid group versus 59.4% placebo treated [adjusted hazard ratio 1.0[95% CI, 0.6–1.8, p=0.99]). Among infants surviving with native liver at one year of age, 8/75 (11%) of infants with successful HPE and 10/25 (40%) of infants with unsuccessful HPE were receiving supplemental nutrition sources including tube feedings and/or total parenteral nutrition (Table 4 online only). Vitamin D status by HPE outcomes is depicted in Table 5 (online only). At 6 month follow-up significantly more infants with unsuccessful HPE 21/42 (50%) had documented vitamin D deficiency than infants with successful HPE 5/75 (7%) (p<0.0001). Table 6 (online only) details the outcomes of infants in both treatment arms at the measurement time points. Measurements after LT were not included in this analysis.
Table 1 details length, weight, and head circumference z-scores at measurement time points for both treatment arms with comparisons to normal and between groups. Z-scores for length were significantly lower than the norms from baseline through 24 months of age for the steroid arm and through 18 months of age for placebo arm. Steroid-treated patients had significantly lower length z-scores at 1, 2, and 3 months post HPE as compared to patients in the placebo arm. Z-scores for weight in both treatment arms were significantly below normal from baseline through 18 months of age in the steroid arm and through 12 months of age in the placebo arm. Patients in the steroid arm had significantly lower weight z-scores than those in the placebo arm from 1 month post HPE throughout the first year of life. Table 7 (online only) details weight to length z-scores, which were significant below normal through 6 months for both groups. The only difference in weight to length z-score between treatment groups was at 6 months (steroid −0.7±1.23 vs placebo −0.43±1.24, p=0.046). Z-scores for head circumference were significantly worse than the norms during the first 6 months post HPE, with patients in the steroid arm significantly lower than the placebo are at 1, 2, and 3 months post HPE.
Table 1.
Length, Weight and Head Circumference (HC) z-scores over time
| Steroid | Placebo | ||||||
|---|---|---|---|---|---|---|---|
| Visit | N | Mean (SD) | P-value compared to standard normal | N | Mean (SD) | P-value compared to standard normal | P-value comparing two arms |
| Length | |||||||
| Baseline | 69 | −0.72 (1.35) | < 0.001 | 67 | −0.55 (1.16) | < 0.001 | 0.7528 |
| 1 Month | 65 | −1.29 (1.25) | < 0.001 | 63 | −0.85 (1.29) | < 0.001 | 0.0241 |
| 2 Month | 65 | −1.51 (1.11) | < 0.001 | 64 | −0.8 (1.12) | < 0.001 | 0.0004 |
| 3 Month | 66 | −1.28 (1.08) | < 0.001 | 62 | −0.87 (1.02) | < 0.001 | 0.0365 |
| 6 Month | 55 | −1.28 (1.19) | < 0.001 | 54 | −0.89 (1.19) | < 0.001 | 0.1721 |
| 1 Year | 50 | −1.13 (1.15) | < 0.001 | 42 | −0.84 (1.19) | < 0.001 | 0.5651 |
| 18 Month | 40 | −0.69 (1.09) | 0.0065 | 34 | −0.71 (1.23) | 0.0032 | 0.9043 |
| 2 Year | 35 | −0.54 (1.24) | 0.0148 | 31 | −0.15 (1.08) | 0.0627 | 0.8255 |
| Weight | |||||||
| Baseline | 70 | −0.83 (1.08) | < 0.001 | 70 | −0.8 (1.06) | < 0.001 | 0.8752 |
| 2 Week | 62 | −1.42 (1.03) | < 0.001 | 62 | −1.15 (0.93) | < 0.001 | 0.0847 |
| 1 Month | 67 | −1.47 (1.01) | < 0.001 | 67 | −1.07 (0.88) | < 0.001 | 0.0015 |
| 2 Month | 67 | −1.48 (1.11) | < 0.001 | 65 | −1.04 (0.9) | < 0.001 | 0.0025 |
| 3 Month | 67 | −1.57 (1.09) | < 0.001 | 64 | −1.04 (0.94) | < 0.001 | 0.0435 |
| 6 Month | 56 | −1.74 (1.18) | < 0.001 | 55 | −1.18 (1.26) | < 0.001 | 0.0256 |
| 1 Year | 50 | −1.41 (1.02) | < 0.001 | 45 | −0.83 (1.47) | < 0.001 | 0.0319 |
| 18 Month | 42 | −0.75 (1.08) | < 0.001 | 35 | −0.33 (1.57) | 0.0822 | 0.0982 |
| 2 Year | 35 | −0.36 (1.03) | 0.1442 | 31 | 0.08 (1.24) | > 0.25 | 0.4736 |
| HC | |||||||
| Baseline | 61 | −1.1 (1.01) | < 0.001 | 61 | −0.87 (1.12) | < 0.001 | 0.8167 |
| 1 Month | 53 | −1.66 (1.31) | < 0.001 | 58 | −0.89 (1.01) | < 0.001 | 0.0013 |
| 2 Month | 58 | −1.55 (0.98) | < 0.001 | 54 | −0.66 (1.34) | < 0.001 | 0.0009 |
| 3 Month | 57 | −1.4 (1.01) | < 0.001 | 58 | −0.65 (1.41) | < 0.001 | 0.0006 |
| 6 Month | 44 | −0.74 (1.04) | 0.0028 | 43 | −0.65 (1.41) | 0.0024 | 0.4246 |
| 1 Year | 45 | −0.2 (1.03) | 0.1286 | 31 | −0.04 (1.38) | > 0.25 | 0.4458 |
| 18 Month | 34 | 0.1 (0.96) | > 0.25 | 29 | 0.18 (1.54) | 0.2306 | 0.6064 |
| 2 Year | 26 | −0.11 (0.87) | > 0.25 | 22 | 0.35 (1.34) | 0.0995 | 0.1790 |
When growth trajectories were compared by treatment status, steroid and placebo arms differed significantly in length (p <0.0001), weight (p=0.009), and head circumference (p<0.0001). Data stratified by HPE outcome was compared to examine differences in treatment effect in those with successful versus unsuccessful HPE. No significant difference was found in length or weight trajectory by treatment group for patients with unsuccessful HPE (Figure 1a). Figure 1b depicts trajectories for patients with successful HPE revealing significantly lower growth trajectory in steroid treated patients compared to placebo patients for both length (p<0.0001) and weight (p=0.047). For length, the difference between treatment groups was most prominent between 30 to 100 days post HPE and diminished thereafter. The difference in weight trajectories between treatment groups appeared to last throughout the first year following HPE. Growth trajectory for head circumference was significantly lower in steroid treated patients in both the successful (p<0.0001) and unsuccessful strata (p<0.0001) early in the time course, with the difference diminishing after 100 days.
Figure 1a and b.
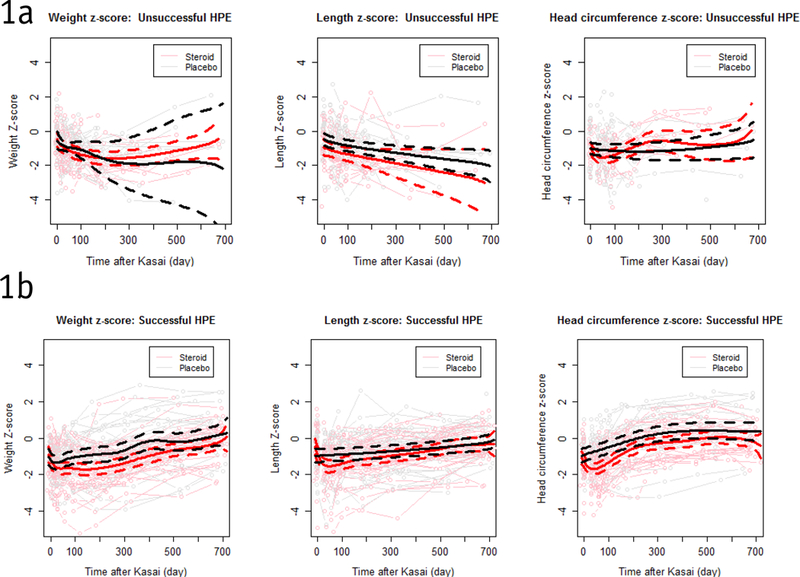
Growth measures curve over time by treatment group for total group with statistical comparison of trend over time stratified by successful HPE – Successful HPE: Solid lines are estimated population mean trajectories; dashed lines show 95% confidence intervals.
HPE = Hepatoportoenterostomy
Mid-arm circumference and triceps skin fold thickness were measured on a sub-set of patients based on availability of trained personnel at the time of each clinical encounter. Steroid therapy was not found to impact z-scores for mid-arm circumference or triceps skin fold thickness (data not shown). Table 2 details data from both treatment arms stratified by HPE outcome status as compared to normal. Muscle mass as measured by these parameters was significantly lower than normal through the first 6 months post HPE regardless of outcome status. In infants with successful HPE, muscle mass recovery occurred during the second 6 months of life, with significantly higher than normal z-scores for mid-arm circumference at 24 months and triceps skin fold thickness at 18 months. Patients with unsuccessful HPE often exited the study following transplant or death, yielding a small sample size beyond one year that limits analysis in this group.
Table 2.
Mid-Arm circumference (MAC) and Triceps skinfold thickness (TSFT) z-scores over time
| Successful HPE | Unsuccessful HPE | |||||
|---|---|---|---|---|---|---|
| Visit | N | Mean (SD) | P-value Compared to standard normal | N | Mean (SD) | P-value Compared to standard normal |
| MAC | ||||||
| Baseline | 7 | −1.35 (1.15) | 0.0178 | 3 | −1.20 (0.19) | 0.0124 |
| 1 Month | 25 | −1.60 (1.29) | < 0.001 | 34 | −1.86 (0.98) | < 0.001 |
| 2 Month | 45 | −1.17 (1.05) | < 0.001 | 39 | −1.60 (1.32) | < 0.001 |
| 3 Month | 56 | −1.17 (1.17) | < 0.001 | 43 | −1.72 (1.12) | < 0.001 |
| 6 Month | 51 | −0.54 (1.28) | 0.0044 | 26 | −1.65 (1.12) | < 0.001 |
| 1 Year | 53 | −0.14 (1.36) | > 0.25 | 13 | −0.89 (0.95) | 0.021 |
| 18 Month | 42 | 0.38 (1.29) | 0.0577 | |||
| 2 Year | 23 | 0.58 (1.06) | 0.0065 | |||
| TSFT | ||||||
| Baseline | 8 | −2.95 (1.10) | < 0.001 | 3 | −2.14 (1.25) | 0.0122 |
| 1 Month | 23 | −2.90 (1.22) | < 0.001 | 30 | −2.33 (1.40) | < 0.001 |
| 2 Month | 42 | −2.17 (1.33) | < 0.001 | 36 | −2.37 (1.52) | < 0.001 |
| 3 Month | 50 | −1.56 (1.55) | < 0.001 | 40 | −2.43 (1.55) | < 0.001 |
| 6 Month | 50 | −0.73 (1.64) | < 0.001 | 24 | −1.50 (1.72) | < 0.001 |
| 1 Year | 51 | −0.19 (1.55) | 0.0358 | 11 | −0.09 (1.59) | > 0.25 |
| 18 Month | 40 | 0.28 (1.52) | 0.0096 | |||
| 2 Year | 21 | 0.51 (1.31) | 0.0542 | |||
MAC = Mid-Arm circumference; HPE = Hepatoportoenterostomy; TSFT = Triceps skinfold thickness
Lastly, we examined the prevalence of linear growth impairment (length < 10%) and linear growth failure (length<5%) at key time intervals in the study stratified by HPE outcome status (Figure 2). At one year of age, despite successful HPE, nearly 40% of patients in both treatment groups were less than the 10th percentile for length. Complete detail on anthropometry z-scores separated by both HPE outcome and treatment group are available in Tables 8 and 9 online only.
Figure 2.
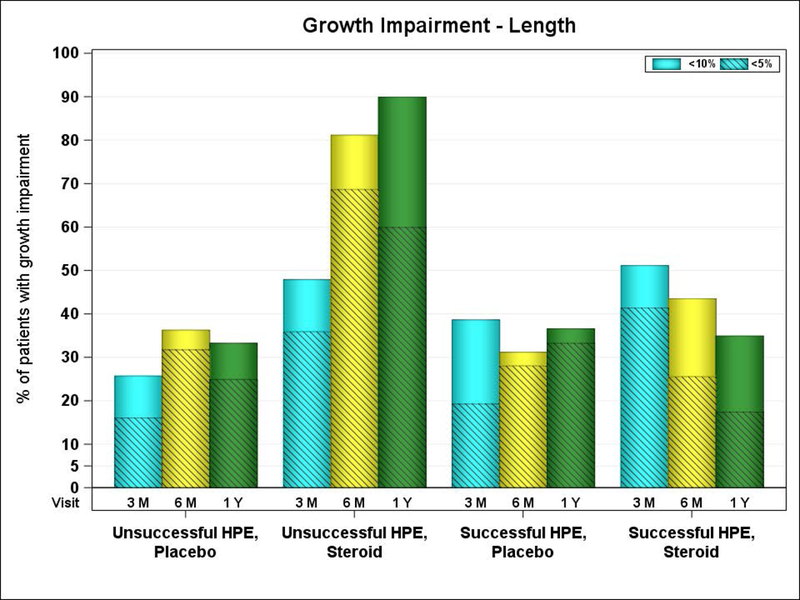
Percentage of growth impairment over time by HPE outcomes.
HPE = Hepatoportoenterostomy
DISCUSSION
This study afforded the opportunity to examine growth longitudinally in infants with BA enrolled in a multi-center clinical trial of steroid therapy. Infants in this trial had significantly lower than normal z-scores for all growth parameters during the first 6 months of life regardless of treatment allocation. Compared to placebo, steroid therapy was associated with significantly lower measurements for length and head circumference during the first three months following HPE, with weight remaining lower in the steroid group until 12 months. Growth trajectory models were used to examine the impact of steroid exposure stratified by HPE outcome. In patients with unsuccessful HPE, growth trajectory for length and weight were abnormal but not significantly impacted by steroid therapy. In patients with successful drainage following HPE, steroid exposure had a strong negative effect on both length and weight, which was most pronounced in the first few months following surgery. Growth trajectory for head circumference was negatively impacted by steroid therapy regardless of HPE outcome. Growth failure was prevalent across all strata of the cohort. The highest rate of growth failure was in steroid treated infants with unsuccessful HPE in which 69% of the steroid treated patients versus 32% of others were below the fifth percentile for length at 6 months.
In this trial, unsuccessful drainage following HPE was strongly associated with failing growth, a finding that is consistent with previous reports (15). Decelerated growth is common in early recovery following HPE, regardless of whether drainage is ultimately effective. However, infants with successful drainage typically have improved growth velocity 3 to 6 months following HPE (15). The results of this trial suggest that if infants with successful HPE are exposed to steroids, recovery of growth velocity will be blunted. Even though the observed catch-up growth may seem reassuring, the consequences of transient growth impairment are not fully understood. In particular, slow head circumference growth during the first three months may be a reflection of impaired brain growth during this vulnerable time in infancy. Without a proven benefit of steroids to improve biliary drainage, it seems difficult to justify the risk of worsening growth impairment during the first year following HPE.
Muscle mass measurements did not vary by treatment allocation. Z-scores for mid-arm circumference and triceps skin fold thickness were significantly below normal through 6 months of age in both the successful and unsuccessful HPE groups. In those with successful HPE, muscle mass recovers to levels equal to or slightly higher than normal at 24 months of age. Reasons for why these infants would have higher than expected z-scores for muscle mass is unclear, but might be related to continued intake of formula with higher caloric density that was initiated during the period of slower growth. Although, the study captured the route of nutritional intake, details regarding caloric intake were not recorded. In those with unsuccessful HPE, the sample size drops considerably after 6 months as children exit the study following LT. Prior to that point, z-scores range from −1.5 to −2.4. Diminished muscle mass may be an early sign of advancing cirrhosis and muscle wasting may increase the risk of frailty. The lower rate of muscle mass data collection as compared to length and weight measures across the timespan of the study highlights the reality that these assessments are not a routine aspect of our clinical examination. Frailty is gaining recognition as an important risk factor for both waiting list and post-transplant morbidity and mortality in adults. (18–20) Assessment of frailty typically includes functional measurements, such as grip strength or a walk test, which are not possible to perform in infants. However, emerging studies in patients with end stage liver disease suggest that sarcopenia, as determined by muscle mass volumes on imaging, correlate well with functional testing. (21) Expanding the routine examination of these patients to include muscle mass measurements using calipers would be a relatively inexpensive and efficient way to screen children for sarcopenia and may lead to earlier identification of children with declining liver function despite adequate bile flow.
Data collection for this trial did not include detailed assessment of nutritional intake. Participants were provided medium chain triglyceride-containing formula to standardize initial formula choice following HPE, but approaches to nutritional support varied across the centers. Supplemental nutritional support, either via tube feedings or parenteral nutrition, was more commonly received by infants with unsuccessful HPE, but nearly 10% of infants with successful outcome were receiving this support at the 6 month follow-up visit. Fat soluble vitamin levels were collected and supplemented per clinical care standards of each site. We report here on vitamin D levels because of the impact on bone metabolism and the potential detrimental effect of deficiency on growth. Patients with unsuccessful HPE were significantly more likely to have measured deficiency of vitamin D. (22, 23) The small number of patients on nutritional therapy did not support analysis to explore associations between supplementation and growth trajectory and muscle mass. Yet, since growth failure and sarcopenia are prevalent problems in the first few months following HPE, focused investigation of optimal nutritional support is an important next step toward maximizing outcomes prior to and following LT.
A significant limitation of this study is related to the degree of incomplete data collection for anthropometric measurements beyond length and weight. Only 84% of visits with length measurements had head circumference measurements and 51% had muscle mass measurements. There may have been bias in which infants received these extended measurements, but it is difficult to predict the direction of the bias. It is possible that infants with more growth deficits were more likely to have been measured, or alternatively they may have been hospitalized or otherwise less available at the indicated follow-up time points. Even with this limitation, this data collection represents the largest reported sample of infants with BA to have standardized muscle mass measurements and provides important insights regarding the evolution of sarcopenia in this population. The findings from this study confirm and extend our previous findings that steroids are not beneficial in BA following HPE. These data should further discourage the ongoing practice of prescribing steroids following HPE.
In summary, analysis of growth measurements collected during the course of the START trial yields several important new observations. Steroid therapy is associated with significantly lower measurements for length, weight, and head circumference during the first six months following HPE, with greatest impact on those with successful bile drainage.
Supplementary Material
Acknowledgments
FUNDING: This work was supported by U01 grants from the National Institute of Diabetes, Digestive and Kidney Diseases (DK 62445 [Mount Sinai School of Medicine], DK 62497 [Cincinnati Children’s Hospital Medical Center], DK 62470 [Children’s Healthcare of Atlanta], DK 62481 [The Children’s Hospital of Philadelphia], DK 62456 [The University of Michigan], DK 84536 [Riley Hospital for Children], DK 84575 [Seattle Children’s Hospital], DK 62500 [UCSF Children’s Hospital], DK 62503 [Johns Hopkins School of Medicine], DK 62466 [Children’s Hospital of Pittsburgh of UPMC], DK 62453 [Children’s Hospital Colorado], DK 62452 [Washington University School of Medicine], DK 84538 [Children’s Hospital Los Angeles], DK 62436 [Ann & Robert H Lurie Children’s Hospital of Chicago], DK103149 [Texas Children’s Hospital], DK103140 [University of Utah]).
The project was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR001878 [The Children’s Hospital of Philadelphia], UL1TR000077 [Cincinnati Children’s Hospital Medical Center, Cincinnati, OH], UL1TR001082 [Children’s Hospital Colorado]. All support is provided without restrictions on publications.
Abbreviations:
- BA
biliary atresia
- CDC
Centers for Disease Control and Prevention
- ChiLDReN
Childhood Liver Disease Research Network
- HC
head circumference
- HPE
Hepatoportoenterostomy
- MAC
Mid-Arm circumference
- RR
relative risk
- START
Steroids in biliary Atresia Randomized
- TSFT
Triceps Skin Fold Thickness
- WHO
World Health Organization
Footnotes
Conflicts of Interest:
The authors have no relevant conflicts of interest to declare
Clinical Trial number & website: ClinicalTrials.gov Study NCT00294684 https://childrennetwork.org/about.aspx
References
- 1.McDiarmid SV, Anand R, Lindblad AS. Studies of Pediatric Liver Transplantation: 2002 update. An overview of demographics, indications, timing, and immunosuppressive practices in pediatric liver transplantation in the United States and Canada. Pediatr Transplant 2004;8:284–94. [DOI] [PubMed] [Google Scholar]
- 2.Shneider BL, Brown MB, Haber B, Whitington PF, Schwarz K, Squires R, et al. A multicenter study of the outcome of biliary atresia in the United States, 1997 to 2000. J Pediatr 2006;148:467–74. [DOI] [PubMed] [Google Scholar]
- 3.Superina R, Magee JC, Brandt ML, Healey PJ, Tiao G, Ryckman F, et al. The anatomic pattern of biliary atresia identified at time of Kasai hepatoportoenterostomy and early postoperative clearance of jaundice are significant predictors of transplant-free survival. Ann Surg 2011;254:577–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokol RJ, Shepherd RW, Superina R, Bezerra JA, Robuck P, Hoofnagle JH. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop. Hepatology (Baltimore, Md) 2007;46(2):566–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bezerra JA, Spino C, Magee JC, Shneider BL, Rosenthal P, Wang KS, et al. Use of corticosteroids after hepatoportoenterostomy for bile drainage in infants with biliary atresia: the START randomized clinical trial. JAMA 2014;311:1750–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucuvalas JC, Horn JA, Slusher J, Alfaro MP, Chernausek SD. Growth hormone insensitivity in children with biliary atresia. J Pediatr Gastroenterol Nutr 1996;23:135–40. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter A, Ng VL, Chapman K, Ling SC, Mouzaki M. Predictive Equations Are Inaccurate in the Estimation of the Resting Energy Expenditure of Children With End-Stage Liver Disease. JPEN J Parenter Enteral Nutr 2017;41:507–11. [DOI] [PubMed] [Google Scholar]
- 8.Chin SE, Shepherd RW, Thomas BJ, Cleghorn GJ, Patrick MK, Wilcox JA, et al. The nature of malnutrition in children with end-stage liver disease awaiting orthotopic liver transplantation. Am J Clin Nutr 1992;56:164–8. [DOI] [PubMed] [Google Scholar]
- 9.Greer RM, Quirk P, Cleghorn GJ, Shepherd RW. Growth hormone resistance and somatomedins in children with end-stage liver disease awaiting transplantation. J Pediatr Gastroenterol Nutr 1998;27:148–54. [DOI] [PubMed] [Google Scholar]
- 10.Quirk P, Owens P, Moyse K, Chin S, Wall C, Ballard J, et al. Insulin-like growth factors I and II are reduced in plasma from growth retarded children with chronic liver disease. Growth Regul 1994;4:35–8. [PubMed] [Google Scholar]
- 11.Barshes NR, Chang IF, Karpen SJ, Carter BA, Goss JA. Impact of pretransplant growth retardation in pediatric liver transplantation. J Pediatr Gastroenterol Nutr 2006;43:89–94. [DOI] [PubMed] [Google Scholar]
- 12.McDiarmid SV, Anand R, Martz K, Millis MJ, Mazariegos G. A multivariate analysis of pre-, peri-, and post-transplant factors affecting outcome after pediatric liver transplantation. Ann Surg 2011;254:145–54. [DOI] [PubMed] [Google Scholar]
- 13.Alonso E, Sheperd R, Martz K, Yin W, Anand R, SPLIT Research Group. Linear Growth Patterns in Pre-Pubertal Children Following Liver Transplantation. Am J Transplant 2009;9:1389–97. [DOI] [PubMed] [Google Scholar]
- 14.Scheenstra R, Gerver WJ, Odink RJ, van Soest H, Peeters PM, Verkade HJ, et al. Growth and final height after liver transplantation during childhood. J Pediatr Gastroenterol Nutr 2008;47:165–71. [DOI] [PubMed] [Google Scholar]
- 15.DeRusso PA, Ye W, Shepherd R, Haber BA, Shneider BL, Whitington PF, et al. Growth failure and outcomes in infants with biliary atresia: a report from the Biliary Atresia Research Consortium. Hepatology (Baltimore, Md) 2007;46:1632–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye W, Lin X, Taylor JM. Semiparametric modeling of longitudinal measurements and time-to-event data--a two-stage regression calibration approach. Biometrics 2008;64:1238–46. [DOI] [PubMed] [Google Scholar]
- 17.Eilers P, Marx B. Flexible Smoothing with B-splines and Penalties. Statist. Sci 1996;11:89–121. [Google Scholar]
- 18.Lai JC, Feng S, Terrault NA, Lizaola B, Hayssen H, Covinsky K. Frailty predicts waitlist mortality in liver transplant candidates. Am J Transplant 2014;14:1870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai JC, Volk ML, Strasburg D, Alexander N. Performance-Based Measures Associate With Frailty in Patients With End-Stage Liver Disease. Transplantation 2016;100:2656–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Underwood PW, Cron DC, Terjimanian MN, Wang SC, Englesbe MJ, Waits SA. Sarcopenia and failure to rescue following liver transplantation. Clin Transplant 2015;29:1076–80. [DOI] [PubMed] [Google Scholar]
- 21.Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol 2014;20:8061–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M, Drug, et al. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 2008;122:398–417. [DOI] [PubMed] [Google Scholar]
- 23.Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 2011;96:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


