Figure 1.
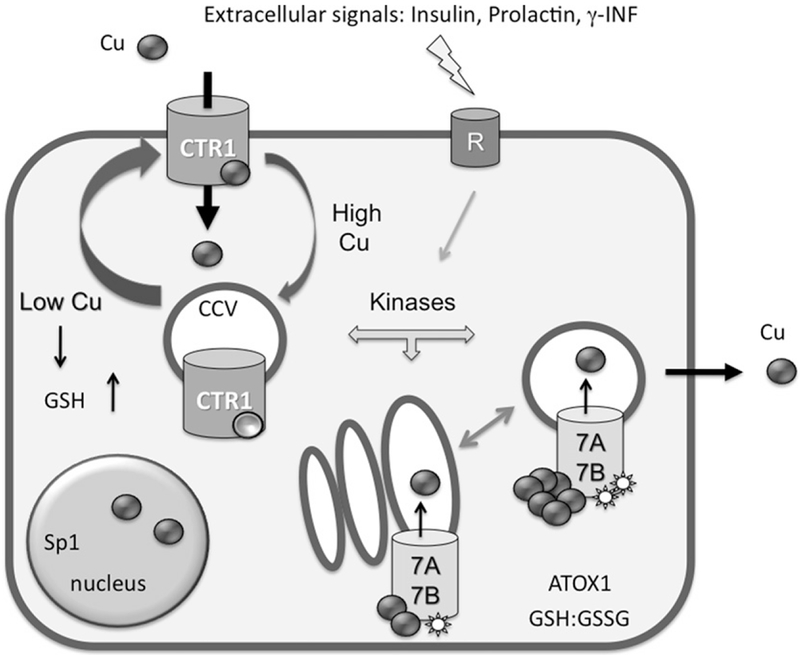
Main regulation modes of copper transporters in human cells. The high-affinity copper transporter hCTR1 is located at the PM, where it facilitates copper entry into the cell. It is currently unknown whether hCTR1 is functional in the intracellular compartments. The cycling between two compartments may be constitutive and involves the clathrin-dependent pathway (CCV, clathrin-coated vesicles). Copper binding to “sensor” sites favors the redistribution of hCTR1 toward intracellular vesicles, whereas copper depletion facilitates the delivery of hCTR1 to the PM. Copper-transporting ATPases 7A and 7B are located in the TGN under basal conditions where they have basal level of phosphorylation (indicated by a star). In response to copper elevation and hormonal signaling, the Cu-ATPases become hyperphosphorylated and traffic to vesicles, where they sequester excess copper. The return to the TGN is linked to the decrease in copper levels and return to a basal level of phosphorylation. Changes in glutathione (GSH) levels and/or alterations of the cellular redox balance GSH:GSSG influence the levels and activity of copper transporters.
