Fig. 4. Oxygen signaling modulates ADL-mediated cold acclimation and thermal responsiveness.
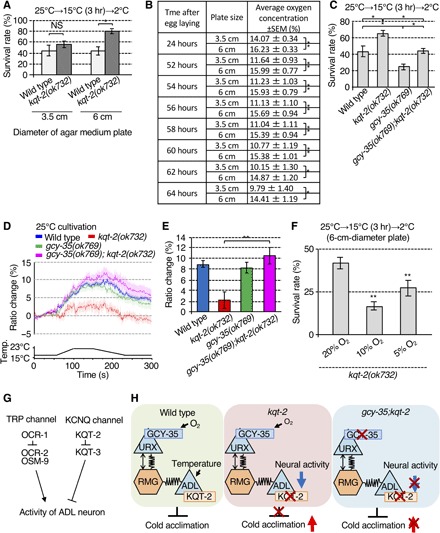
(A) Cold acclimation of animals cultivated on 3.5- and 6.0-cm-diameter plates. Number of assays ≥ 8. Error bar indicates SEM. Comparisons were performed using the Tukey-Kramer method. *P < 0.05, **P < 0.01. (B) Oxygen concentration at the surface of 3.5- and 6.0-cm-diameter agar plates with bacterial lawns and worms cultured from 24 to 64 hours after egg laying. Larger 6.0-cm-diameter plates exhibit higher sustained O2 concentrations. For each point, n = 10. Error bar indicates SEM. Comparisons were performed using the unpaired t test (Welch). *P < 0.05, **P < 0.01. (C) gcy-35 mutant, lacking the intracellular O2 sensor, suppresses the supranormal cold acclimation of kqt-2(ok732). Number of assays ≥ 11. Error bar indicates SEM. Comparisons were performed using the Tukey-Kramer method. *P < 0.05, **P < 0.01. (D and E) Abrogation of Ca2+ thermal response in ADL neurons of kqt-2(ok732) is suppressed in kqt-2(ok732);gcy-35(ok769). n ≥ 18. Error bar indicates SEM. Comparisons were performed using the Tukey-Kramer method. *P < 0.05, **P < 0.01. The bar graph indicates the average ratio change from 160 to 170 s (E). Wild-type data in (D) are shared with Figs. 2 (C, E, I, and K) and 3 (D, F, and H) and fig. S6A, given that the experiments were conducted simultaneously (D). kqt-2(ok723) data in (D) are shared with Figs. 2 (E and K) and 3H, given that the experiments were conducted simultaneously (D). (F) Enhanced cold acclimation of kqt-2 was recovered to a normal level after cultivation under 5% O2. Number of assays ≥ 9. Error bar indicates means SEM. Comparisons were performed using Dunnett’s test. *P < 0.05, **P < 0.01. (G) Model of molecular mechanisms regulating activity in ADL neurons. OCR-1 acts as a negative regulator of OCR-2/OSM-9 thermally responsive TRP channels in ADL neurons. KQT-2 acts as a negative regulator of KQT-3 potassium channels. (H) Model of neuronal circuitry integrating ADL temperature sensing with oxygen signaling by URX visceral oxygen sensory neurons via RMG hub interneurons. In wild-type worms, URX neurons may indirectly modulate temperature signaling of ADL neurons in cold acclimation. Left: Sensing of high O2 levels by URX neurons, which is dependent on GCY-35, is signaled through RMG to ADL neurons via chemical and electrical synapses to promote cold acclimation, presumably by inhibiting ADL excitability. Middle: Loss of KQT-2 in ADL neurons results in decreased neuronal activity in response to temperature change and increased cold acclimation. Loss of GCY-35 blocks active O2 signaling from URX neurons, mimicking a low O2 environment. This increases ADL excitability sufficiently to suppress the inhibitory effect of KQT-2 loss of function. Right: Supranormal cold acclimation observed in kqt-2 mutants is thus suppressed by gcy-35 mutations or low O2 concentration.
