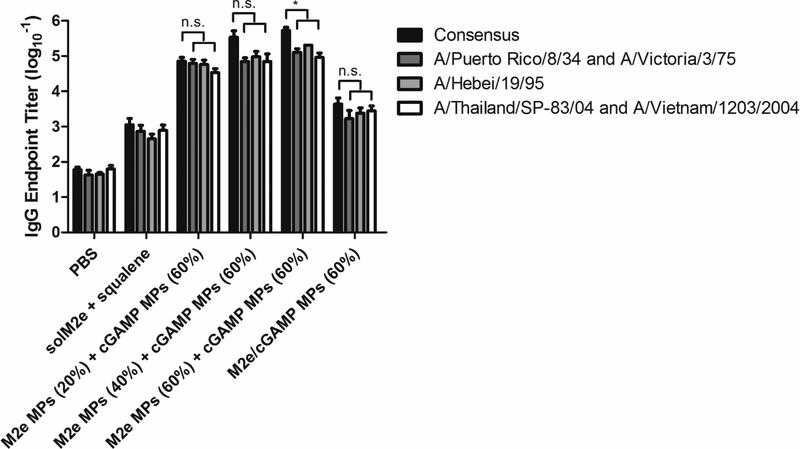
Figure 7.
Total IgG antibody titers against M2e sequences containing mismatch mutations to the consensus sequence. 1 mismatch (A/Puerto Rico/8/34 and A/Victoria/3/75), 2 mismatches (A/Hebei/19/95), and 3 mismatches (A/Thailand/SP-83/04 and A/Vietnam/1203/2004). Serum was collected on Day 28 after mice were vaccinated on Day 0 and 21 with phosphatebuffered saline (PBS), soluble M2e + MF59-like AddaVax (solM2e + squalene), M2e-loaded microparticles (M2e MPs (20, 40, or 60%)) + cGAMP-loaded microparticles (cGAMP MPs (60%)), or MPs containing both M2e and cGAMP (M2e/cGAMP MPs (60%)). All MPs were composed of acetalated dextran (Ace-DEX), and percentages indicate Ace-DEX MP relative cyclic acetal coverages. Pertinent groups received 10 μg M2e and 1 μg cGAMP. Statistical significance is presented as *p < 0.05, performed using a one-way ANOVA. Data are presented as mean ± standard error of the mean (n = 5).