Abstract
Gene expression regulation is the result of complex interactions between transcriptional and post-transcriptional controls, resulting in cell-type-specific gene expression patterns that are determined by the developmental and differentiation stage of pathophysiological conditions. Understanding the complexity of gene expression regulatory networks is fundamental to gene therapy, an approach which has the potential to treat and cure inherited disorders by delivering the correct gene to patient specific cells or tissues by means of both viral and non-viral vectors. Besides the issues of biosafety, in recent years efforts have focused on achieving a robust and sustained transgene expression, which attains a phenotypic correction in several diseases, while avoiding transgene-related adverse effects, such as overexpression-associated cytotoxicity and/or immune responses to the transgene. In this sense, the use of cell-type-specific promoters and microRNA target sequences (miRTs) in gene transfer expression cassettes have allowed for a restricted expression after gene transfer in several studies. This review will focus on the use of transcriptional and post-transcriptional regulation to achieve a highly specific and safe transgene expression, as well as their application in ex vivo and in vivo gene therapeutic approaches.
Main Text
Regulation of Gene Expression and Gene Therapy
For molecular biology, gene expression is defined as the transcription of a gene into mRNA followed by its translation into protein. Despite the simplicity of this definition, the regulation of gene expression is a highly orchestrated event starting within the gene promoter, a region that binds RNA polymerase II and the general transcription factors (GTFs). This interaction involves the participation of several elements, including enhancers, silencers, insulators, and tethering elements. Among these elements, enhancers and their transcription factors play a pivotal role in initiating the gene expression.1
Additional members involved in gene expression regulation are the small non-coding RNAs or microRNAs, which are found at the post-transcriptional level. MicroRNAs, or miRNAs, are short RNA sequences that range from 17 to 24 bp in length, and they are involved in the post-transcriptional regulation by binding to the 3′ or the 5′ UTR of their target mRNAs.2 Interaction of miRNAs with their mRNA targets regulates gene expression via mRNA degradation and/or translational repression.2 The miRNAs play a pivotal role in several cellular processes, such as development, differentiation, proliferation, and apoptosis3, 4 and are expressed in a specific manner and at determined levels depending on the tissue, cell type, lineage, or differentiation state.
Overall, gene expression regulation is the result of complex interactions between transcriptional and post-transcriptional controls, which depend on developmental stage, cell type, and pathophysiological conditions.1, 5 Understanding the complexity of gene expression regulatory networks has a tremendous impact not only from a biological perspective, but more so with respect to translational medicine. By taking advantage of this knowledge, gene therapy has the potential to treat critical diseases by restoring gene expression to the natural site of synthesis and at physiological levels. This offers the possibility of achieving a long-term expression within a therapeutic window, while avoiding adverse reactions, such as cell stress or toxicity due to transgene overexpression6 or immune responses triggered by ubiquitous or non-specific expression.7
The delivery of the correct gene to patient cells or tissues occurs by means of both viral and non-viral vectors. Among the viral vectors available for gene transfer, lentiviral vectors (LVs) have been extensively used for gene delivery in research, pre-clinical studies, and in clinical trials.8, 9, 10, 11, 12
This review will focus on transcriptional and post-transcriptional regulation strategies for cell-type-specific transgene expression as well as their application for ex vivo and in vivo gene transfer approaches using LVs.
Lentiviral Vectors
More than 20 years ago, Naldini et al.13 proposed the use of the human immunodeficiency virus type 1 (HIV-1) for the development of LVs. Such vectors are one of the best tools available in the effort to develop efficient viral vectors for gene therapy. Indeed, these LVs present several characteristics that make them very attractive, such as (1) an active transport mechanism to translocate the genomic material into the nucleus regardless of the cell cycle status, making them able to transduce both dividing and non-dividing cells; (2) a lack of viral protein expression after transduction; (3) accommodation of expression cassettes of up 10 kb; (4) a low or absent genotoxicity; and (5) a sustained transgene expression after transduction due to their genome-integration ability.8, 14, 15 These characteristics result in several applications for LVs, including transgene overexpression,16, 17 sustained silencing of genes,18 immunization,19, 20 cancer cell targeting,21, 22 in vivo molecular imaging,23 stem cell induction and/or modification,24, 25 and gene editing.26, 27
An important aspect in the generation of HIV-derived LVs is the biosafety concerns related to their derivation, among which is the rare possibility of forming replication-competent lentiviruses.8 LVs are classified into “generations” based on the packaging constructs used for vector production. The current version is the third generation, which achieves enhanced safety by splitting sequences and genes necessary for viral production into four different plasmids. This design makes the generation of wild-type HIV by recombination highly improbable.15 The transfer construct containing the expression cassette does not contain genes coding for viral proteins, but only cis-acting sequences of HIV: the packaging sequence ψ, necessary to recruit the vector genome RNA to be packaged into the viral particle; a central polypurine tract (PPT) sequence, a nuclear translocation signal; and RRE, the Rev response element important for unspliced RNA transport to the cytoplasm.28, 29 Biosafety has been improved with the introduction of self-inactivating (SIN) transfer constructs.30 Unlike the retroviral long terminal repeat (LTR) sequences that are subjected to silencing by DNA methylation, SIN LVs tend to be more robustly expressed.31
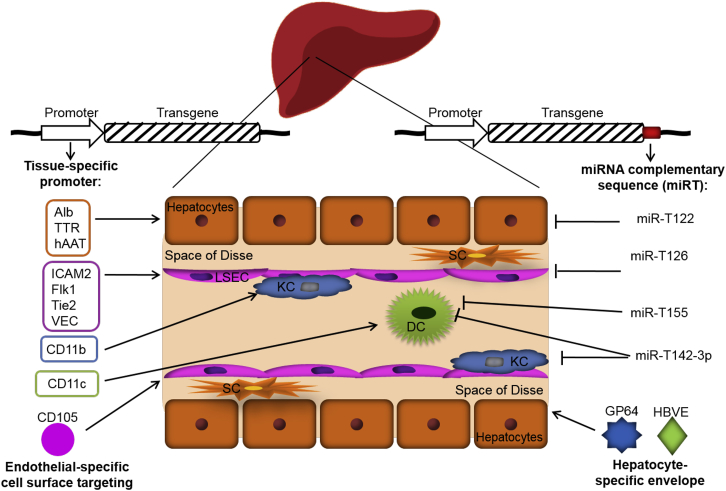
Besides the improvement of packaging and transfer constructs, the natural HIV envelope glycoprotein has been replaced with glycoproteins from other unrelated viruses in a process called “pseudotyping.” The most commonly used is the glycoprotein G from the vesicular stomatitis virus (VSV-G), which confers to LVs a greater stability and wider cellular tropism. This, however, represents an obstacle for in vivo applications, especially when the transgene is toxic for transduced cells, such as pro-apoptotic, oncogene, or suicide genes.32 On the other hand, a wider tropism could be useful in an application aiming to trigger an antigen-specific immune response, such as in the case of cancer gene therapy, by efficiently transducing antigen-presenting cells (APCs). This, however, is a condition to avoid in the case of gene therapy in which transgene expression, aiming to treat the pathological phenotype, could trigger an immunological response against the transgene itself.33 Pantropic transgene expression can be averted with the use of other viral glycoproteins, for example, the envelope proteins of hepatitis B virus and the baculovirus GP64, which allow hepatocyte-restricted LV entry (Figure 1).34
Figure 1.
Cell-Type-Specific Targeting Approaches
The drawing shows a schematic reproduction of a liver sinusoid. Liver sinusoidal endothelial cells (LSECs) are cells delineating the hepatic sinusoids. Hepatocyes are separated from endothelial cells by the space of Disse. Stellate cells (SCs) are contained in the space of Disse. On the side facing the bloodstream are found hepatic macrophages, also known as Kupffer cells (KCs), in tight contact with LSECs, and dendritic cells (DCs). Liver-directed gene transfer can be achieved using vector containing cell-type-specific promoter, such as albumin (Alb), Transthyretin (TTR), human alpha antitrypsin (hAAT) promoters for hepatocytes, and ICAM2, Flk1, Tie2, and VEC for endothelial cells. Envelopes of viral vectors can be modified to restrict vector entry to specific cell types, such as the GP64 glycoprotein from baculovirus and hepatitis B virus envelope (HBVE) for hepatocyte transductional targeting. The specificity of transgene expression can be further increased using target sequences with perfect complementarity to cell-specific microRNA (miRT), such as miRT-122 for hepatocytes, miRT-126 for endothelial cells, or miRT-142-3p and miRT-155 for hematopoietic cells, and specifically suppressing the transgene expression in defined cell types without affecting the expression in other cells. ICAM2, intercellular adhesion molecule 2; Flk1, fetal liver kinase 1, the VEGF receptor; Tie2, angiopoietin receptor; VEC, VE-cadherin.
To restrict transgene expression in desired cell types after vector delivery, it is possible to apply several approaches. The main strategies to achieve this goal are three: (1) transcriptional regulation using cell-type-specific promoters; (2) post-transcriptional regulation, or transgene de-targeting, taking advantage of the expression of different miRNAs in certain cell types; (3) restriction of transduction by means of glycoproteins derived from several viruses which confer LVs to the tropism for the desired cell types (Figure 1). This review will focus on the first two strategies, while restriction of transduction is reviewed by others in this issue.
Transcriptional Regulation
To obtain a robust transgene expression following gene transfer, several promoters with or without the addition of enhancer sequences can be inserted into an expression cassette of a gene transfer vector. The promoter is often a strong constitutive promoter, such as the cytomegalovirus (CMV), the spleen focus-forming virus (SFFV), or the human phosphoglycerate kinase (PGK) promoter.32 While ensuring a ubiquitous transgene expression, these promoters show several drawbacks. Among these are that (1) these promoters are subject to promoter inactivation more than cell-type-specific ones;35, 36 (2) they are stronger activators of the defense machinery in the host cells, resulting in cytokine-induced promoter inactivation;37 (3) they have an increased probability to cause insertional mutagenesis due to their enhancer activity;36 and (4) the ubiquitous expression, including in the transduced APCs, most often results in the development of an immune response against the transgene.38 For these reasons, scientists have developed several strategies to obtain cell-type-specific transgene expression. The use of these promoters is aimed at selectively targeting transgene expression to desired locations, allowing a prolonged gene expression (Figure 1). Several studies have demonstrated that restricting transgene expression in particular target cells by specific promoters results in sustained transgene expression that is useful for disease treatments, while the ubiquitous expression can trigger immune reactions against the transgene products.38, 39
The liver cells, in particular hepatocytes, represent a typical example. These cells are a good target for transgene expression since they produce and secrete large amounts of proteins.40 Moreover, hepatocytes’ ability to induce tolerance toward antigens makes them more appealing for gene therapeutic approaches.41, 42, 43 Taking advantage of the liver tolerogenic ability, Akbarpour et al.44 suggested that hepatocyte-targeted gene therapy could have a role in protecting against type 1 diabetes (T1D). By expressing an immunodominant epitope of T cells, the insulin B chain 9-23 (InsB9-23), under the control of a hepatocyte-specific chimeric promoter (enhanced transthyretin [ET]), they were able to induce InsB9-23-specific regulatory T cells (Tregs) and to revert the T1D condition by the combination of in vivo gene transfer and anti-CD3 treatment.44 The hepato-specific transgene expression was also able to favor the conversion of ovalbumin (OVA)-specific naive CD4+ T cells, adoptively transferred into naive recipient mice before the administration of an OVA-containing LV, into OVA-specific induced Tregs (iTregs).7 Using the ET promoter for the production of the factor IX (FIX) of the coagulation cascade, Cantore and colleagues7, 12, 45 were able to correct the bleeding phenotype in hemophilia B mice and dogs. Moreover, this approach prevented the immune response to the transgene and reverted the anti-transgene pre-existing immunity by tolerance induction in hemophilia B mice.45 Despite these promising results, the same strategy applied for the expression of coagulation factor VIII (FVIII) was not effective in preventing the formation of neutralizing antibodies against the transgene in treated hemophilia A mice.34 Recently, it has been shown that hepatocyte-restricted FVIII expression following adeno-associated vector (AAV) delivery is associated with an anti-FVIII immune response in a dose-dependent manner and with cellular stress in hemophilic mice.6 These results can be explained by the fact that hepatocytes are not the primary source of FVIII production. However, more recent pre-clinical studies for hemophilia A (HA) employed several AAV serotypes and several combinations of liver-specific promoters and enhancer elements driving the expression of FVIII transgene: Greig et al.46 observed different effects after vector administration in mice and in non-human primates47 ranging from none to high titer of anti-FVIII antibody formation without a direct correlation between the peak of FVIII expression and the formation of antibodies.46 Moreover, in a recent clinical trial from BioMarin, hepatocyte-directed FVIII gene therapy resulted in therapeutic levels of FVIII activity (19%–164%) 52 weeks after vector delivery in patients receiving a high dose of AAV with no evidence of anti-FVIII antibody or inhibitor formation.48 In the past, hepatocytes have been considered the main source of FVIII, since an orthotopic liver transplantation of a normal liver into a hemophilic dog resulted in a complete correction of the bleeding phenotype.49 More recent studies from our and other groups have shown that FVIII is secreted by endothelial cells, mainly liver sinusoidal endothelial cells (LSEC),50, 51, 52 and to a lesser extent by hematopoietic cells.52, 53 By restricting FVIII expression to endothelial cells, largely LSEC, using a LV platform containing the transgene under the control of the vascular endothelial cadherin (VEC) promoter, our group has recently treated the bleeding phenotype in three different strains of immunocompetent hemophilia A mice and prevented the formation of anti-FVIII immune responses after immunization with FVIII several weeks after gene transfer. Further, this strategy was able to revert inhibitor titers in hemophilia A mice previously immunized with FVIII, while the depletion of Tregs in treated mice resulted in a temporary loss of tolerance with the formation of anti-FVIII antibodies, suggesting a role for LSEC in FVIII-specific tolerance induction.54 These studies indicate that optimal transgene expression is achieved in tissues or cell types physiologically involved in the production of the coagulation protein, thus reaching phenotypic correction of the disease and possibly avoiding immune responses against the transgene.
Several cell-type-specific promoters used in LV expression cassettes in a number of in vivo studies have obtained promising results for the treatment of diseases. Recently, the treatment of Alzheimer’s disease (AD) with the expression of the neuroprotective protein secreted amyloid precursor protein-alpha (sAPPα) under the control of the neuron specific synapsin 1 promoter was able to prevent the development of the AD phenotype and to rescue the synaptic plasticity in a mouse model of the disease.55
Synthetic Promoters for Transcriptional Regulation
Another strategy for transcriptional regulation is the design of synthetic promoters that are carried out to combine regulatory elements with particular characteristics in vitro, and which are not predictive of their efficiency in vivo.56 Computational methods, which consider evolutionary conserved transcription factor binding sites (TFBSs) related to strong tissue-specific expression and their co-occurrence with other TFBSs, can represent an alternative strategy for the generation of cell-type-specific promoters with a high efficiency. This approach was used by Chuah et al.56 for the identification of cis-acting regulatory modules (CRMs) containing TFBS clusters associated with a robust hepatocyte-specific expression. They designed an AAV for FIX liver-specific gene therapy that contained a hepatocyte-specific promoter, with either a strong (transthyretin, TTR) or moderate (paralemmin, Palm) activity, in combination with hepatocyte-specific (HS) CRMs. The addition of these hepatocyte-specific-CRMs, especially hepatocyte-specific-CRM8, resulted in an improved FIX expression in vivo, increasing the transcription from both TTR and Palm promoters and correcting the bleeding phenotype in hemophilia B mice. This system also produced therapeutic levels of human FIX (20%–35%) in non-human primates while maintaining tissue selectivity; however, a few weeks after injection, they observed reduced FIX levels with a concomitant production of anti-FIX antibodies.56 Although this approach was able to restrict tissue specificity for transgene production, it resulted in a supraphysiological expression that could have led to hepatocyte stress and consequently an immune response to the transgene, as observed for hepatocyte-specific FVIII overexpression.6 We are aware that the immune system is a very complex system and that many factors are responsible for the immune responses observed after gene therapy, which should be taken into account. These include the type of vector used for gene transfer, the viral proteins from the viral vectors, vector dosage and route of administration, transgene immunogenicity, and species-specific transgenes. This topic, however, is beyond the scope of the present review and have been recently discussed elsewhere.57, 58, 59, 60
Transcriptional Regulation for Cancer Gene Therapy
For cancer treatment, several gene therapy strategies have been used. It has long been known that tumor growth and metastasis depend on angiogenesis in a process involving endothelial cell activation, proliferation, migration, and sprouting.61 Consequently, the use of endothelial-specific promoters, such as angiopoietin receptor (Tie2), vascular endothelial growth factor receptor (VEGFR) 1 and 2, von Willebrand factor (vWF), and other promoters, driving the production of genes that are blocking the activation, proliferation, migration, and reorganization of endothelial cells, have been shown to be effective both in vitro and in vivo, affecting the tumor vasculature and consequently diminishing the tumor growth.62
Another possible strategy for cancer gene therapy is targeting directly the tumor cells. The human telomerase reverse transcriptase (hTERT), a component of telomerase, is expressed in most malignant tumors, but not in most normal somatic cells. Yu et. al63 used the hTERT promoter to express the cytosine deaminase (CD) suicide gene and the reporter gene GFP in a bicistronic LV expression cassette. After intratumoral LV delivery into tumor-bearing nude mice, they observed tumor growth suppression following administration of 5-fluorocytosine (5-FC) and, at the same time, they were able to monitor the tumor growth and the therapeutic efficacy of the treatment by in vivo imaging by monitoring the GFP expression in cancer cells. This strategy, however, was not effective for hTERT negative tumors.
Finally, cancer immunotherapy is an effective tool for stimulating the immune system to react against cancer cells by obtaining the expression of tumor antigens under the control of APC-specific promoters, such as the HLA-DR or the dectin-2 promoters.64, 65 Lopes et al.,65 for example, showed that intravenous and subcutaneous injection of an LV containing the human melanoma antigen NY-ESO-1 under the control of the dectin-2 promoter was able to induce transgene expression in splenic dectin-2+ dendritic cells (DCs) and in CD11c+ DCs in lymph nodes close to the subcutaneous injection point, resulting in a strong NY-ESO-1-specific CD4+ and CD8+ T cell response and providing a safe and effective vaccine by targeting antigen expression to DCs.
Transcriptional Targeting after Ex Vivo Gene Therapy
Ex vivo gene therapy, by the means of a combination of stem cell manipulation and gene transfer techniques, allows the use of autologous cells instead of cells from allogenic donors. Hematopoietic stem cell (HSC) transduction is a typical example of advances made in gene therapy for clinical applications. In a recent study, Doering et al.66 developed an LV platform containing a bioengineered FVIII with enhanced expression under the control of the CD68 (GP110, macrosialin, or LAMP4) promoter, restricting FVIII expression in myeloid cells for the transduction of autologous HSC CD34+ that upon differentiation in monocytes were able to increase FVIII production and secretion. Even though they do not clearly show FVIII expression by HSC CD34+-derived monocytes-macrophages in vivo, which would demonstrate the specificity of CD68 promoter, they do demonstrate the feasibility of HSC gene therapy for the treatment of hemophilia A.
Since the gene transfer occurs ex vivo, some of the issues related to the in vivo delivery of viral vector, such as whole-body distribution and triggering of innate antiviral responses upon systemic administration, can be disregarded. Concerns regarding insertional mutagenesis using integrating viral vectors are, however, still present, especially with the use of strong ubiquitous promoters and/or enhancers that may transactivate neighboring genes and cause malignant transformation.67 This is even more relevant for the treatment of patients that are cancer prone, as in the case of individuals affected by Wiscott-Aldrich syndrome (WAS).10 WAS is an X-linked disease caused by mutations in the gene encoding WASP, a protein involved in cytoskeleton regulation.10 Patients with WAS suffer mainly from eczema, thrombocytopenia, immune deficiency, and susceptibility to infections as a consequence of immunodeficiency, and their pathological status can be cured by LV-transduced HSC autologous transplantation. Reports from two clinical trials10, 68 showed resolution from eczema and susceptibility to infections and an improvement in platelet counts, immune function, and clinical score in all treated patients. Most importantly, no adverse events related to the genotoxicity due to the use of the LV was observed, unlike the first gene therapy clinical trial using a Moloney-leukemia-virus-derived vector (MLV).68 These results suggest that integrating LV-mediated HSC gene therapy is a safe treatment for WAS. In recent studies, the WASP transgene was inserted into a SIN LV under the transcriptional control of WAS promoter, ensuring transgene expression and function in a physiological manner due to its natural gene regulation.10 In pre-clinical studies, WASP expression under the control of the WAS promoter was able to restore IL-2 production and proliferation upon T cell receptor (TCR) activation in WAS T cells, as well as cytoskeleton reorganization in WAS DC. WASP expression was restored both in murine Lin− and human CD34+ HSC without detrimental effects on cell growth, survival, and differentiation.9, 69, 70, 71
Restriction of transgene expression in desired cell types requires the combination of additional enhancer elements to obtain a more selective promoter. For example, two different groups reported the use of this strategy for the treatment of X-linked chronic granulomatous disease (X-CGD), a primary immunodeficiency due to mutations in the CYBB gene encoding for the catalytic subunit gp91phox of the phagocyte nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase.72, 73 Santilli et al.72 created a synthetic promoter containing myeloid TFBSs by fusing the 446-bp proximal promoter of c-Fes downstream of the 360-bp sequence of the cathepsin G minimal promoter. The resulting chimeric promoter was exploited to drive the expression of gp91phox in an LV used to transduce X-CGD murine Lin− and human CD34+ HSCs before transplantation in gp91phox-deficient mice and nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice, respectively. With this chimeric promoter, they obtained high-level transgene expression in committed myeloid cells and granulocytes, restoring normal NADPH-oxidase activity. For the same purpose, Chiriaco et al.73 used a myeloid-specific promoter (MSP) composed of a 1.5-kb minimal promoter sequence from the gp91phox locus fused to the SP146 synthetic enhancer and promoter in an LV expression cassette. After transplantation of human transduced CD34+ HSCs in a mouse model of X-CGD, they restored gp91phox expression and function in differentiated myeloid cells.73 In fact, the myeloid-specific promoter maintained the regulation of transgene expression during bone marrow (BM) development and considerably reduced gp91phox expression in CD34+ HSCs, thus minimizing the risk of genotoxicity and potential perturbation of reactive oxygen species levels. Consequently, the use of myeloid-specific promoters within an LV cassette were shown to be promising for the treatment of X-CGD with improved safety and efficacy compared to the SFFV-based γ-retroviral vector used in a previous clinical trial showing enhancer-mediated mutagenesis and diminution of effectiveness over time due to silencing of the viral LTR.36
In some cases, the genetic elements necessary to ensure high and cell-type-specific transgene expression upon gene transfer require a complex design. LVs can be used to transfer complicated and bulky structures, as in the case of β-globin gene correction for the treatment of severe sickle cell disease (SCD) and β-thalassemia. The design of the LV for human β-globin gene therapy, to achieve high and erythroid-specific expression, requires not only the β-globin gene containing a mutation (βA(T87Q)) with anti-sickling properties, but also β-globin gene introns, promoter, enhancers, and β-locus control region (β-LCR).74, 75 Phase 1/2 and phase 3 studies to evaluate safety and efficacy of this approach are ongoing, with no adverse events reported so far.76, 77, 78, 79
Transcriptional Regulated Inducible Promoters
Inducible promoters are also a valid option when it is necessary to control or induce transgene expression. Inducible promoters provide an additional regulatory point, as they can be induced under specific conditions and in selected tissues. The most common inducible systems are based on tetracyclin (Tet) or its more potent analog doxycycline (Dox).32 In vivo uses of these systems, however, are limited due to their leakage. This limitation has been bypassed by the construction of single inducible LV containing the transactivator under the control of a cell-type-specific promoter, such as VEC promoter for endothelial cells80 or the albumin (Alb) promoter for hepatocytes,81 with the transgene under the Tet-inducible promoter. Finally, concerns using Dox are related to the possible development of resistance to the antibiotic, the toxicity of the inducer molecule, and, despite the absence of immunogenicity in different mouse strains, the development of immune responses to the chimeric transactivators, as reported in studies with intramuscularly delivered Tet-ON activators in non-human primates.32
Post-transcriptional Regulation
Transgene expression restricted to a desired tissue or cell type by using cell-type-specific promoters alone, although successful in some applications, does present some weaknesses. These include difficulties in truly identifying and reconstructing the promoter of a gene and the fact that unique cell-specific transcriptional patterns belong to very few genes, while different promoters can be active in different cell types or conditions. A possible approach to overcome this issue takes advantage of the RNA interference (RNAi) machinery present in cells, thus adding a post-transcriptional layer of control to suppress transgene expression in particular cell types. RNAi and associated small RNA-mediated processes induce gene silencing through transcription reduction, caused by mRNA destabilization, or translation inhibition in a sequence-specific fashion.82 This mechanism of silencing was first described in 1993,83 and in early 2000 these small RNAs, called microRNA or miRNA, were recognized as a distinct class of regulators with important biological functions.84, 85, 86 Since they are highly conserved between species, miRNA expression and biologic functional studies can be carried on in animal models. Moreover, the expression of different miRNAs has been found to be altered in several pathological conditions, such as cancer87, 88 and heart89, 90 and liver91 diseases, thus rendering them diagnostic and/or prognostic biomarkers as well as potential therapeutic targets for several pathologies.
By taking advantage of adding specific miRNA target sequences (miRTs), which are perfectly complementary to the desired miRNAs, it is possible to increase the cell-type specificity and stringency of transgene expression not only in desired cell types, but also in certain cells at a determined differentiation stage and/or pathological condition (Figure 1).
To benefit from this post-transcriptional regulation system, several factors need to be taken into account for the selection of candidate miRNA: (1) the cell-type-specific expression; (2) the level of expression needed to reach a certain threshold; (3) the miRT configuration, in terms of perfect complementarity (exclusivity with respect to other miRNA) and numbers of repetition of the sequence (usually 2–4 are sufficient); (4) the order of insertion and the length of spacer sequences when miRTs are used in combination.4
Brown et al.92 were the first to use this strategy in vivo to de-target transgene expression in hematopoietic cells, including APCs. They demonstrated a stable gene transfer in immunocompetent mice by adding to the 3′ of their LV expression cassette target sequences for the 3p strand of miR-142 (miRT-142-3p). However, the same strategy applied for FVIII expression for the gene therapy of hemophilia A using a ubiquitous promoter and miR-142 was not able to prevent the formation of immune responses against the transgenes.7, 54 Since then, several studies using different vector systems and disease models have shown encouraging results using this miRNA-dependent post-transcriptional regulation strategy.4 Naldini’s group12, 45, 93 added to the 3′ UTR of their LV expression cassette, containing the hepatocyte-specific ET promoter, the miRT-142-3p to avoid transgene expression in APCs, obtaining sustained transgene expression and correction in murine and canine models of hemophilia B.
When a slight leakage of a cell-type-specific promoter is observed with transgene expression in unwanted cell types, it is possible to use more than one miRT to restrict transgene expression in desired cell types. Our group recently showed that FVIII expression following in vivo delivery of an LV containing the FVIII transgene under the transcriptional control of the endothelial-specific VEC promoter was able to correct the bleeding phenotype of hemophilia A mice.54 In preliminary experiments, however, we observed transgene expression not only in endothelial cells, but also in a few hepatocytes and in hepatic and splenic hematopoietic cells (mainly macrophages). Thus, we further increased endothelial-specific transgene expression by adding the miRT-142-3p and miRT-122 sequences to the endothelial-specific LV expression cassette, de-targeting transgene expression in hematopoietic cells and hepatocytes, respectively.54 As such, the addition of more post-transcriptional control sequences can efficiently overcome off-target problems.
The differential miRNA expression in different cell subtypes can offer the opportunity to target a specific cell type, avoiding the expression in subpopulations. For example, the miRNA-126 is a miRNA expressed mainly in endothelial cells94, 95 and in hematopoietic stem and progenitor cells (HSPCs).96 Chiarico et al.73 showed the efficacy of HSC gene therapy in a mouse model of X-CGD by combining transcriptional, using a myeloid-specific promoter (SP146.gp91), and post-transcriptional regulation, using the miRT-126, in an LV platform. With this strategy, they were able to express gp91phox in myeloid cells avoiding transgene expression in human and murine HSPCs and to restore gp91phox expression in myeloid cells in X-CGD mice after HSC gene therapy.
Similarly, in a cancer gene therapeutic approach, Escobar et al.97 expressed interferon-α in tumor-infiltrating monocytes-macrophages by combining the use of the Tie2 enhancer and promoter in combination with miRTs for HSPC miRNAs, miR-126 and miR-130a, in an LV for HSC gene therapy to avoid transgene expression in HSCs and inhibit breast cancer progression. More recently, miRNA-126 expression was also reported in plasmacytoid DCs (pDCs).98 Our group has targeted FVIII expression in myeloid cells using an LV containing the transgene under the transcriptional control of the myeloid-specific CD11b (integrin alpha M, Mac-1) promoter, with or without the addition of miRT-126 as a post-transcriptional level of control to avoid transgene expression in endothelial cells and pDCs. When injected in vivo, the miRT-126 efficiently de-targeted transgene expression in endothelial cells and in pDCs without affecting the expression in other myeloid cells, including conventional DCs, and surprisingly avoided immune responses against the transgene product. While further investigations are necessary, this suggests a possible role for pDCs as APCs in the adaptive immune response against FVIII triggered by transgene expression.54
Recently, Keavaney et al.99 described the use of miRTs in association to the use of the pan-neuronal human synapsin 1 promoter (hSyn) to target specifically interneurons with a high level of target selectivity (91% ± 3%), highlighting the potential of miRNA-based viral gene targeting to specific neuron subtypes.
Downregulation of transgene expression is particularly important in gene-therapy approaches involving suicide genes such as cancer gene therapy, where transgene expression is required in cancer cells avoiding the expression in normal cells as an essential prerequisite. Thus, differential miRNAs expression in cancer cells offers an effective method for avoiding off-target effects in normal cells. Dhungel et al.100 recently exploited this approach to target transgene expression in hepatocellular carcinoma (HCC) cell lines taking advantage of downregulation of miRNA 122a and miRNA 199a in these cells. Hepatocytes express both miRNA 122a and miRNA 199a, while miRNA 199a is expressed by other liver cell types, such as hepatic stellate cells and LSECs. By adding the target sequences for miRNA 122a and miRNAs 199a at the 3′ UTR of the transgene, they were able to avoid transgene expression in normal hepatocytes while obtaining robust expression in HCC cell lines, showing the feasibility of preventing suicide gene therapy off-target effects by sparing non-HCC hepatic cells.
Conclusions
LVs have been shown to be efficient delivery vectors for several transgenes in virtually any cell type, thus opening their use to several therapeutic applications, especially for the field of gene therapy. Their ability to transduce both dividing and non-dividing cells and to integrate in the genome of transduced cells offers the possibility to approach numerous diseases that cannot be treated by other vectors. Further, the reduced incidence of immunity against LV elements compared to other viral vectors and reduced genotoxicity make these vectors optimal candidates for complementary approaches to existing gene therapy strategies that, while efficient in transgene expression, can still present some limitations.
Despite the ability of some target tissues to induce tolerance to certain transgenes,44 there is an increasing evidence demonstrating that the best target for the treatment of a disease is the cell type producing the specific protein, such as FIX in hepatocytes or FVIII in endothelial cells. This cell-type-specific expression results in long-term expression without triggering the immune system against the transgene and possibly reverting the pre-existing immunity against the exogenous protein.45, 54 Transcriptional targeting with the use of cell-type-specific promoters, gives the opportunity to express the transgene in a physiological manner, despite some limitations, such as promoter leakage.
The discovery of miRNA-regulated gene expression has opened new potentials to the use of LVs in gene therapy. Taking advantage of the endogenous gene silencing system and the cell-type-specific expression of determined miRNAs, it is now possible to avoid or silence transgene expression in certain cell types at the post-transcriptional level by adding short repeated miRT. The use of miRTs alone, however, is insufficient to achieve specific expression or to avoid immune reaction against the transgene. Further, the use of miRTs in combination with strong ubiquitous promoters, specifically viral promoters such as CMV and SFFV, may result in miRT overexpression and in the downregulation of involved miRNAs. In turn, this may lead to a loss of regulation of the natural mRNA targets and consequently interference with the gene expression profile of involved cells.101 As such, the combination of transcriptional and post-transcriptional targeting considerably enhances the specificity of the expression cassette in LV constructs, offering the possibility to target not only particular cell types, but also determined subpopulations both ex vivo and in vivo (Figure 1). Moreover, these targeting strategies are not restricted to LVs and are suitable for the design of other viral (e.g., AAV) and non-viral (e.g., plasmid DNA-loaded nanoparticle) vectors employed in several gene transfer approaches.
The additional use of other targeting strategies, such as the transductional targeting with envelope modification for cell-type-specific transduction (i.e., pseudotyping; Figure 1) and the genomic targeting, with site-specific insertion, would further improve the specificity of transgene expression and the safety profile of LVs, consequently increasing the potential of LV-based gene therapy for the treatment of an increasing number of diseases.
Author Contributions
A.F. and S.M. conceived the structure of the article, wrote the paper, and prepared the figure.
Conflicts of Interest
The authors declare no conflict of interest.
Acknowledgments
We thank Dr. Gillian Walker and Dr. Chiara Borsotti for English revision and critical reading of the manuscript. This work was supported by ERC 261178 and Horizon 2020 (hemAcure project #66742) to A.F.
References
- 1.Spitz F., Furlong E.E.M. Transcription factors: from enhancer binding to developmental control. Nat. Rev. Genet. 2012;13:613–626. doi: 10.1038/nrg3207. [DOI] [PubMed] [Google Scholar]
- 2.Catalanotto C., Cogoni C., Zardo G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016;17:E1712. doi: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi Y.J., Lin C.P., Risso D., Chen S., Kim T.A., Tan M.H., Li J.B., Wu Y., Chen C., Xuan Z. Deficiency of microRNA miR-34a expands cell fate potential in pluripotent stem cells. Science. 2017;355:eaag1927. doi: 10.1126/science.aag1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geisler A., Fechner H. MicroRNA-regulated viral vectors for gene therapy. World J. Exp. Med. 2016;6:37–54. doi: 10.5493/wjem.v6.i2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandra S., Vimal D., Sharma D., Rai V., Gupta S.C., Chowdhuri D.K. Role of miRNAs in development and disease: Lessons learnt from small organisms. Life Sci. 2017;185:8–14. doi: 10.1016/j.lfs.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Lange A.M., Altynova E.S., Nguyen G.N., Sabatino D.E. Overexpression of factor VIII after AAV delivery is transiently associated with cellular stress in hemophilia A mice. Mol. Ther. Methods Clin. Dev. 2016;3:16064. doi: 10.1038/mtm.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mátrai J., Cantore A., Bartholomae C.C., Annoni A., Wang W., Acosta-Sanchez A., Samara-Kuko E., De Waele L., Ma L., Genovese P. Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk. Hepatology. 2011;53:1696–1707. doi: 10.1002/hep.24230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Milone M.C., O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scaramuzza S., Biasco L., Ripamonti A., Castiello M.C., Loperfido M., Draghici E., Hernandez R.J., Benedicenti F., Radrizzani M., Salomoni M. Preclinical safety and efficacy of human CD34(+) cells transduced with lentiviral vector for the treatment of Wiskott-Aldrich syndrome. Mol. Ther. 2013;21:175–184. doi: 10.1038/mt.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aiuti A., Biasco L., Scaramuzza S., Ferrua F., Cicalese M.P., Baricordi C., Dionisio F., Calabria A., Giannelli S., Castiello M.C. Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science. 2013;341:1233151. doi: 10.1126/science.1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biffi A., Montini E., Lorioli L., Cesani M., Fumagalli F., Plati T., Baldoli C., Martino S., Calabria A., Canale S. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science. 2013;341:1233158. doi: 10.1126/science.1233158. [DOI] [PubMed] [Google Scholar]
- 12.Cantore A., Ranzani M., Bartholomae C.C., Volpin M., Valle P.D., Sanvito F., Sergi L.S., Gallina P., Benedicenti F., Bellinger D. Liver-directed lentiviral gene therapy in a dog model of hemophilia B. Sci. Transl. Med. 2015;7:277ra28. doi: 10.1126/scitranslmed.aaa1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naldini L., Blömer U., Gallay P., Ory D., Mulligan R., Gage F.H., Verma I.M., Trono D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 14.Annoni A., Gregori S., Naldini L., Cantore A. Modulation of immune responses in lentiviral vector-mediated gene transfer. Cell. Immunol. 2018 doi: 10.1016/j.cellimm.2018.04.012. Published online April 27, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Follenzi A., Naldini L. Generation of HIV-1 derived lentiviral vectors. Methods Enzymol. 2002;346:454–465. doi: 10.1016/s0076-6879(02)46071-5. [DOI] [PubMed] [Google Scholar]
- 16.López-Ornelas A., Mejía-Castillo T., Vergara P., Segovia J. Lentiviral transfer of an inducible transgene expressing a soluble form of Gas1 causes glioma cell arrest, apoptosis and inhibits tumor growth. Cancer Gene Ther. 2011;18:87–99. doi: 10.1038/cgt.2010.54. [DOI] [PubMed] [Google Scholar]
- 17.Wang L., Song J., Zhang J., Zhu C., Ma Y., Xu X. Lentiviral vector-mediate ATG3 overexpression inhibits growth and promotes apoptosis of human SKM-1 cells. Mol. Biol. Rep. 2014;41:2093–2099. doi: 10.1007/s11033-014-3058-0. [DOI] [PubMed] [Google Scholar]
- 18.Hutson T.H., Foster E., Moon L.D.F., Yáñez-Muñoz R.J. Lentiviral vector-mediated RNA silencing in the central nervous system. Hum. Gene Ther. Methods. 2014;25:14–32. doi: 10.1089/hgtb.2013.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H., Zhang L., Kung S.K.P. Emerging applications of lentiviral vectors in dendritic cell-based immunotherapy. Immunotherapy. 2010;2:685–695. doi: 10.2217/imt.10.44. [DOI] [PubMed] [Google Scholar]
- 20.Oldham R.A., Berinstein E.M., Medin J.A. Lentiviral vectors in cancer immunotherapy. Immunotherapy. 2015;7:271–284. doi: 10.2217/imt.14.108. [DOI] [PubMed] [Google Scholar]
- 21.Fan G., Bo J., Wan R., Peng M., Luan Y., Deng M., Xu L. The effect of lentiviral vector-mediated RNA interference targeting hypoxia-inducible factor 1α on the uptake of fluorodeoxyglucose ((18)f) in the human pancreatic cancer cell line, patu8988. Cancer Biother. Radiopharm. 2015;30:160–168. doi: 10.1089/cbr.2014.1700. [DOI] [PubMed] [Google Scholar]
- 22.Zhou J., Li B., Peng C., Wang F., Fu Z., Zhou C., Hong D., Ye F., Lü W., Xie X. Inhibition of cervical cancer cell growth in vitro and in vivo by lentiviral-vector mediated shRNA targeting the common promoter of HPV16 E6 and E7 oncogenes. Antiviral Res. 2013;98:305–313. doi: 10.1016/j.antiviral.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee S., De A. Applications of lentiviral vectors in molecular imaging. Front. Biosci. 2014;19:835–853. doi: 10.2741/4251. [DOI] [PubMed] [Google Scholar]
- 24.Hu X., Xie P., Li W., Li Z., Shan H. Direct induction of hepatocyte-like cells from immortalized human bone marrow mesenchymal stem cells by overexpression of HNF4α. Biochem. Biophys. Res. Commun. 2016;478:791–797. doi: 10.1016/j.bbrc.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez-Danés A., Consiglio A., Richaud Y., Rodríguez-Pizà I., Dehay B., Edel M., Bové J., Memo M., Vila M., Raya A., Izpisua Belmonte J.C. Efficient generation of A9 midbrain dopaminergic neurons by lentiviral delivery of LMX1A in human embryonic stem cells and induced pluripotent stem cells. Hum. Gene Ther. 2012;23:56–69. doi: 10.1089/hum.2011.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lombardo A., Genovese P., Beausejour C.M., Colleoni S., Lee Y.L., Kim K.A., Ando D., Urnov F.D., Galli C., Gregory P.D. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 2007;25:1298–1306. doi: 10.1038/nbt1353. [DOI] [PubMed] [Google Scholar]
- 27.Blasco R.B., Karaca E., Ambrogio C., Cheong T.C., Karayol E., Minero V.G., Voena C., Chiarle R. Simple and rapid in vivo generation of chromosomal rearrangements using CRISPR/Cas9 technology. Cell Rep. 2014;9:1219–1227. doi: 10.1016/j.celrep.2014.10.051. [DOI] [PubMed] [Google Scholar]
- 28.Follenzi A., Ailles L.E., Bakovic S., Geuna M., Naldini L. Gene transfer by lentiviral vectors is limited by nuclear translocation and rescued by HIV-1 pol sequences. Nat. Genet. 2000;25:217–222. doi: 10.1038/76095. [DOI] [PubMed] [Google Scholar]
- 29.Zennou V., Petit C., Guetard D., Nerhbass U., Montagnier L., Charneau P. HIV-1 genome nuclear import is mediated by a central DNA flap. Cell. 2000;101:173–185. doi: 10.1016/S0092-8674(00)80828-4. [DOI] [PubMed] [Google Scholar]
- 30.Zufferey R., Dull T., Mandel R.J., Bukovsky A., Quiroz D., Naldini L., Trono D. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J. Virol. 1998;72:9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum. Gene Ther. 2005;16:1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- 32.Goyvaerts C., Liechtenstein T., Bricogne C., Escors D., Breckpot K. Targeted lentiviral vectors: current applications and future potential. In: Martin F., editor. Gene Therapy: Tools and Potential Applications. IntechOpen; 2013. [Google Scholar]
- 33.Annoni A., Battaglia M., Follenzi A., Lombardo A., Sergi-Sergi L., Naldini L., Roncarolo M.G. The immune response to lentiviral-delivered transgene is modulated in vivo by transgene-expressing antigen-presenting cells but not by CD4+CD25+ regulatory T cells. Blood. 2007;110:1788–1796. doi: 10.1182/blood-2006-11-059873. [DOI] [PubMed] [Google Scholar]
- 34.Matsui H., Hegadorn C., Ozelo M., Burnett E., Tuttle A., Labelle A., McCray P.B., Jr., Naldini L., Brown B., Hough C., Lillicrap D. A microRNA-regulated and GP64-pseudotyped lentiviral vector mediates stable expression of FVIII in a murine model of Hemophilia A. Mol. Ther. 2011;19:723–730. doi: 10.1038/mt.2010.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Prösch S., Stein J., Staak K., Liebenthal C., Volk H.D., Krüger D.H. Inactivation of the very strong HCMV immediate early promoter by DNA CpG methylation in vitro. Biol. Chem. Hoppe Seyler. 1996;377:195–201. doi: 10.1515/bchm3.1996.377.3.195. [DOI] [PubMed] [Google Scholar]
- 36.Stein S., Ott M.G., Schultze-Strasser S., Jauch A., Burwinkel B., Kinner A., Schmidt M., Krämer A., Schwäble J., Glimm H. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat. Med. 2010;16:198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- 37.Liu B.H., Wang X., Ma Y.X., Wang S. CMV enhancer/human PDGF-β promoter for neuron-specific transgene expression. Gene Ther. 2004;11:52–60. doi: 10.1038/sj.gt.3302126. [DOI] [PubMed] [Google Scholar]
- 38.Annoni A., Brown B.D., Cantore A., Sergi L.S., Naldini L., Roncarolo M.G. In vivo delivery of a microRNA-regulated transgene induces antigen-specific regulatory T cells and promotes immunologic tolerance. Blood. 2009;114:5152–5161. doi: 10.1182/blood-2009-04-214569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Follenzi A., Battaglia M., Lombardo A., Annoni A., Roncarolo M.G., Naldini L. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- 40.Sun P., Zhou X., Farnworth S.L., Patel A.H., Hay D.C. Modeling human liver biology using stem cell-derived hepatocytes. Int. J. Mol. Sci. 2013;14:22011–22021. doi: 10.3390/ijms141122011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crispe I.N. Hepatocytes as Immunological Agents. J. Immunol. 2016;196:17–21. doi: 10.4049/jimmunol.1501668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.LoDuca P.A., Hoffman B.E., Herzog R.W. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr. Gene Ther. 2009;9:104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sack B.K., Herzog R.W., Terhorst C., Markusic D.M. Development of gene transfer for induction of antigen-specific tolerance. Mol. Ther. Methods Clin. Dev. 2014;1:14013. doi: 10.1038/mtm.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Akbarpour M., Goudy K.S., Cantore A., Russo F., Sanvito F., Naldini L., Annoni A., Roncarolo M.G. Insulin B chain 9-23 gene transfer to hepatocytes protects from type 1 diabetes by inducing Ag-specific FoxP3+ Tregs. Sci. Transl. Med. 2015;7:289ra81. doi: 10.1126/scitranslmed.aaa3032. [DOI] [PubMed] [Google Scholar]
- 45.Annoni A., Cantore A., Della Valle P., Goudy K., Akbarpour M., Russo F., Bartolaccini S., D’Angelo A., Roncarolo M.G., Naldini L. Liver gene therapy by lentiviral vectors reverses anti-factor IX pre-existing immunity in haemophilic mice. EMBO Mol. Med. 2013;5:1684–1697. doi: 10.1002/emmm.201302857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Greig J.A., Wang Q., Reicherter A.L., Chen S.J., Hanlon A.L., Tipper C.H., Clark K.R., Wadsworth S., Wang L., Wilson J.M. Characterization of Adeno-Associated Viral Vector-Mediated Human Factor VIII Gene Therapy in Hemophilia A Mice. Hum. Gene Ther. 2017;28:392–402. doi: 10.1089/hum.2016.128. [DOI] [PubMed] [Google Scholar]
- 47.Greig J.A., Nordin J.M.L., White J.W., Wang Q., Bote E., Goode T., Calcedo R., Wadsworth S., Wang L., Wilson J.M. Optimized Adeno-Associated Viral-Mediated Human Factor VIII Gene Therapy in Cynomolgus Macaques. Hum. Gene Ther. 2018 doi: 10.1089/hum.2018.080. Published online July 23, 2018. [DOI] [PubMed] [Google Scholar]
- 48.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-Factor VIII Gene Transfer in Severe Hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 49.Storb R., Marchioro T.L., Graham T.C., Willemin M., Hougie C., Thomas E.D. Canine hemophilia and hemopoietic grafting. Blood. 1972;40:234–238. [PubMed] [Google Scholar]
- 50.Shahani T., Covens K., Lavend’homme R., Jazouli N., Sokal E., Peerlinck K., Jacquemin M. Human liver sinusoidal endothelial cells but not hepatocytes contain factor VIII. J. Thromb. Haemost. 2014;12:36–42. doi: 10.1111/jth.12412. [DOI] [PubMed] [Google Scholar]
- 51.Follenzi A., Benten D., Novikoff P., Faulkner L., Raut S., Gupta S. Transplanted endothelial cells repopulate the liver endothelium and correct the phenotype of hemophilia A mice. J. Clin. Invest. 2008;118:935–945. doi: 10.1172/JCI32748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zanolini D., Merlin S., Feola M., Ranaldo G., Amoruso A., Gaidano G., Zaffaroni M., Ferrero A., Brunelleschi S., Valente G. Extrahepatic sources of factor VIII potentially contribute to the coagulation cascade correcting the bleeding phenotype of mice with hemophilia A. Haematologica. 2015;100:881–892. doi: 10.3324/haematol.2014.123117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Follenzi A., Raut S., Merlin S., Sarkar R., Gupta S. Role of bone marrow transplantation for correcting hemophilia A in mice. Blood. 2012;119:5532–5542. doi: 10.1182/blood-2011-07-367680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merlin S., Cannizzo E.S., Borroni E., Bruscaggin V., Schinco P., Tulalamba W., Chuah M.K., Arruda V.R., VandenDriessche T., Prat M. A Novel Platform for Immune Tolerance Induction in Hemophilia A Mice. Mol. Ther. 2017;25:1815–1830. doi: 10.1016/j.ymthe.2017.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tan V.T.Y., Mockett B.G., Ohline S.M., Parfitt K.D., Wicky H.E., Peppercorn K., Schoderboeck L., Yahaya M.F.B., Tate W.P., Hughes S.M., Abraham W.C. Lentivirus-mediated expression of human secreted amyloid precursor protein-alpha prevents development of memory and plasticity deficits in a mouse model of Alzheimer’s disease. Mol. Brain. 2018;11:7. doi: 10.1186/s13041-018-0348-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chuah M.K., Petrus I., De Bleser P., Le Guiner C., Gernoux G., Adjali O., Nair N., Willems J., Evens H., Rincon M.Y. Liver-specific transcriptional modules identified by genome-wide in silico analysis enable efficient gene therapy in mice and non-human primates. Mol. Ther. 2014;22:1605–1613. doi: 10.1038/mt.2014.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brown B.D., Lillicrap D. Dangerous liaisons: the role of “danger” signals in the immune response to gene therapy. Blood. 2002;100:1133–1140. doi: 10.1182/blood-2001-11-0067. [DOI] [PubMed] [Google Scholar]
- 58.Ge Y., Powell S., Van Roey M., McArthur J.G. Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood. 2001;97:3733–3737. doi: 10.1182/blood.v97.12.3733. [DOI] [PubMed] [Google Scholar]
- 59.Zhou H.S., Liu D.P., Liang C.C. Challenges and strategies: the immune responses in gene therapy. Med. Res. Rev. 2004;24:748–761. doi: 10.1002/med.20009. [DOI] [PubMed] [Google Scholar]
- 60.Arruda V.R., Samelson-Jones B.J. Gene therapy for immune tolerance induction in hemophilia with inhibitors. J. Thromb. Haemost. 2016;14:1121–1134. doi: 10.1111/jth.13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Folkman J. Toward an understanding of angiogenesis: search and discovery. Perspect. Biol. Med. 1985;29:10–36. doi: 10.1353/pbm.1985.0049. [DOI] [PubMed] [Google Scholar]
- 62.Dong Z., Nör J.E. Transcriptional targeting of tumor endothelial cells for gene therapy. Adv. Drug Deliv. Rev. 2009;61:542–553. doi: 10.1016/j.addr.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu S.-T., Li C., Lü M.H., Liang G.P., Li N., Tang X.D., Wu Y.Y., Shi C.M., Chen L., Li C.Z. Noninvasive and real-time monitoring of the therapeutic response of tumors in vivo with an optimized hTERT promoter. Cancer. 2012;118:1884–1893. doi: 10.1002/cncr.26476. [DOI] [PubMed] [Google Scholar]
- 64.Cui Y., Golob J., Kelleher E., Ye Z., Pardoll D., Cheng L. Targeting transgene expression to antigen-presenting cells derived from lentivirus-transduced engrafting human hematopoietic stem/progenitor cells. Blood. 2002;99:399–408. doi: 10.1182/blood.v99.2.399. [DOI] [PubMed] [Google Scholar]
- 65.Lopes L., Dewannieux M., Gileadi U., Bailey R., Ikeda Y., Whittaker C., Collin M.P., Cerundolo V., Tomihari M., Ariizumi K., Collins M.K. Immunization with a lentivector that targets tumor antigen expression to dendritic cells induces potent CD8+ and CD4+ T-cell responses. J. Virol. 2008;82:86–95. doi: 10.1128/JVI.01289-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Doering C.B., Denning G., Shields J.E., Fine E.J., Parker E.T., Srivastava A., Lollar P., Spencer H.T. Preclinical development of a hematopoietic stem and progenitor cell bioengineered factor VIII lentiviral vector gene therapy for hemophilia A. Hum. Gene Ther. 2018;29:1183–1201. doi: 10.1089/hum.2018.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rivière I., Dunbar C.E., Sadelain M. Hematopoietic stem cell engineering at a crossroads. Blood. 2012;119:1107–1116. doi: 10.1182/blood-2011-09-349993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hacein-Bey Abina S., Gaspar H.B., Blondeau J., Caccavelli L., Charrier S., Buckland K., Picard C., Six E., Himoudi N., Gilmour K. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA. 2015;313:1550–1563. doi: 10.1001/jama.2015.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marangoni F., Bosticardo M., Charrier S., Draghici E., Locci M., Scaramuzza S., Panaroni C., Ponzoni M., Sanvito F., Doglioni C. Evidence for long-term efficacy and safety of gene therapy for Wiskott-Aldrich syndrome in preclinical models. Mol. Ther. 2009;17:1073–1082. doi: 10.1038/mt.2009.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Charrier S., Dupré L., Scaramuzza S., Jeanson-Leh L., Blundell M.P., Danos O., Cattaneo F., Aiuti A., Eckenberg R., Thrasher A.J. Lentiviral vectors targeting WASp expression to hematopoietic cells, efficiently transduce and correct cells from WAS patients. Gene Ther. 2007;14:415–428. doi: 10.1038/sj.gt.3302863. [DOI] [PubMed] [Google Scholar]
- 71.Dupré L., Marangoni F., Scaramuzza S., Trifari S., Hernández R.J., Aiuti A., Naldini L., Roncarolo M.G. Efficacy of gene therapy for Wiskott-Aldrich syndrome using a WAS promoter/cDNA-containing lentiviral vector and nonlethal irradiation. Hum. Gene Ther. 2006;17:303–313. doi: 10.1089/hum.2006.17.303. [DOI] [PubMed] [Google Scholar]
- 72.Santilli G., Almarza E., Brendel C., Choi U., Beilin C., Blundell M.P., Haria S., Parsley K.L., Kinnon C., Malech H.L. Biochemical correction of X-CGD by a novel chimeric promoter regulating high levels of transgene expression in myeloid cells. Mol. Ther. 2011;19:122–132. doi: 10.1038/mt.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chiriaco M., Farinelli G., Capo V., Zonari E., Scaramuzza S., Di Matteo G., Sergi L.S., Migliavacca M., Hernandez R.J., Bombelli F. Dual-regulated lentiviral vector for gene therapy of X-linked chronic granulomatosis. Mol. Ther. 2014;22:1472–1483. doi: 10.1038/mt.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weber L., Poletti V., Magrin E., Antoniani C., Martin S., Bayard C., Sadek H., Felix T., Meneghini V., Antoniou M.N. An Optimized Lentiviral Vector Efficiently Corrects the Human Sickle Cell Disease Phenotype. Mol. Ther. Methods Clin. Dev. 2018;10:268–280. doi: 10.1016/j.omtm.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwiatkowski J.L., Thompson A.A., Rasko J., Hongeng S., Schiller G.J., Anurathapan U., Cavazzana M., Ho P.J., von Kalle C., Kletzel M. Clinical Outcomes up to 3 Years Following Lentiglobin Gene Therapy for Transfusion-Dependent β-Thalassemia in the Northstar Hgb-204 Study. Blood. 2017;130:360. [Google Scholar]
- 77.Kanter J., Walters M.C., Hsieh M.M., Krishnamurti L., Kwiatkowski J., Kamble R.T., von Kalle C., Kuypers F.A., Cavazzana, Leboulch P. Interim Results from a Phase 1/2 Clinical Study of Lentiglobin Gene Therapy for Severe Sickle Cell Disease. Blood. 2016;128:1176. [Google Scholar]
- 78.Kanter J., Walters M.C., Hsieh M., Krishnamurti L., Kwiatkowski J.L., Kamble R., von Kalle C., Joseney-Antoine M., Pierciey F.J., Jr., Shi W. Interim Results from a Phase 1/2 Clinical Study of Lentiglobin Gene Therapy for Severe Sickle Cell Disease. Blood. 2017;130:527. [Google Scholar]
- 79.Walters M.C., Rasko J., Hongeng S., Kwiatkowski J., Schiller G.J., Kletzel M., Ho P.J., Vichinsky E., von Kalle C., Cavazzana M. Update of Results from the Northstar Study (HGB-204): A Phase 1/2 Study of Gene Therapy for Beta-Thalassemia Major Via Transplantation of Autologous Hematopoietic Stem Cells Transduced Ex-Vivo with a Lentiviral Beta AT87Q-Globin Vector (LentiGlobin BB305 Drug Product) Blood. 2015;126:201. [Google Scholar]
- 80.Yang G., Kramer M.G., Fernandez-Ruiz V., Kawa M.P., Huang X., Liu Z., Prieto J., Qian C. Development of Endothelial-Specific Single Inducible Lentiviral Vectors for Genetic Engineering of Endothelial Progenitor Cells. Sci. Rep. 2015;5:17166. doi: 10.1038/srep17166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bai J., Li J., Mao Q. Construction of a single lentiviral vector containing tetracycline-inducible Alb-uPA for transduction of uPA expression in murine hepatocytes. PLoS ONE. 2013;8:e61412. doi: 10.1371/journal.pone.0061412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fischer S.E.J. RNA Interference and MicroRNA-Mediated Silencing. Curr. Protoc. Mol. Biol. 2015;112:26.1.1–26.1.5. doi: 10.1002/0471142727.mb2601s112. [DOI] [PubMed] [Google Scholar]
- 83.Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 84.Lee R.C., Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 85.Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 86.Lau N.C., Lim L.P., Weinstein E.G., Bartel D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 87.Naro Y., Ankenbruck N., Thomas M., Tivon Y., Connelly C.M., Gardner L., Deiters A. Small Molecule Inhibition of MicroRNA miR-21 Rescues Chemosensitivity of Renal-Cell Carcinoma to Topotecan. J. Med. Chem. 2018;61:5900–5909. doi: 10.1021/acs.jmedchem.7b01891. [DOI] [PubMed] [Google Scholar]
- 88.Sakha S., Muramatsu T., Ueda K., Inazawa J. Exosomal microRNA miR-1246 induces cell motility and invasion through the regulation of DENND2D in oral squamous cell carcinoma. Sci. Rep. 2016;6:38750. doi: 10.1038/srep38750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yan H., Ma F., Zhang Y., Wang C., Qiu D., Zhou K., Hua Y., Li Y. miRNAs as biomarkers for diagnosis of heart failure: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96:e6825. doi: 10.1097/MD.0000000000006825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong L.L., Wang J., Liew O.W., Richards A.M., Chen Y.-T. MicroRNA and Heart Failure. Int. J. Mol. Sci. 2016;17:502. doi: 10.3390/ijms17040502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schueller F., Roy S., Vucur M., Trautwein C., Luedde T., Roderburg C. The Role of miRNAs in the Pathophysiology of Liver Diseases and Toxicity. Int. J. Mol. Sci. 2018;19:E261. doi: 10.3390/ijms19010261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brown B.D., Venneri M.A., Zingale A., Sergi Sergi L., Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat. Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 93.Brown B.D., Cantore A., Annoni A., Sergi L.S., Lombardo A., Della Valle P., D’Angelo A., Naldini L. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 2007;110:4144–4152. doi: 10.1182/blood-2007-03-078493. [DOI] [PubMed] [Google Scholar]
- 94.Fish J.E., Santoro M.M., Morton S.U., Yu S., Yeh R.F., Wythe J.D., Ivey K.N., Bruneau B.G., Stainier D.Y., Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev. Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang S., Aurora A.B., Johnson B.A., Qi X., McAnally J., Hill J.A., Richardson J.A., Bassel-Duby R., Olson E.N. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev. Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gentner B., Visigalli I., Hiramatsu H., Lechman E., Ungari S., Giustacchini A., Schira G., Amendola M., Quattrini A., Martino S. Identification of hematopoietic stem cell-specific miRNAs enables gene therapy of globoid cell leukodystrophy. Sci. Transl. Med. 2010;2:58ra84. doi: 10.1126/scitranslmed.3001522. [DOI] [PubMed] [Google Scholar]
- 97.Escobar G., Moi D., Ranghetti A., Ozkal-Baydin P., Squadrito M.L., Kajaste-Rudnitski A., Bondanza A., Gentner B., De Palma M., Mazzieri R., Naldini L. Genetic engineering of hematopoiesis for targeted IFN-α delivery inhibits breast cancer progression. Sci. Transl. Med. 2014;6:217ra3. doi: 10.1126/scitranslmed.3006353. [DOI] [PubMed] [Google Scholar]
- 98.Agudo J., Ruzo A., Tung N., Salmon H., Leboeuf M., Hashimoto D., Becker C., Garrett-Sinha L.A., Baccarini A., Merad M., Brown B.D. The miR-126-VEGFR2 axis controls the innate response to pathogen-associated nucleic acids. Nat. Immunol. 2014;15:54–62. doi: 10.1038/ni.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Keaveney M.K., Tseng H.A., Ta T.L., Gritton H.J., Man H.Y., Han X. A MicroRNA-Based Gene-Targeting Tool for Virally Labeling Interneurons in the Rodent Cortex. Cell Rep. 2018;24:294–303. doi: 10.1016/j.celrep.2018.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dhungel B., Ramlogan-Steel C.A., Steel J.C. Synergistic and independent action of endogenous microRNAs 122a and 199a for post-transcriptional liver detargeting of gene vectors. Sci. Rep. 2018;8:15539. doi: 10.1038/s41598-018-33801-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gentner B., Schira G., Giustacchini A., Amendola M., Brown B.D., Ponzoni M., Naldini L. Stable knockdown of microRNA in vivo by lentiviral vectors. Nat. Methods. 2009;6:63–66. doi: 10.1038/nmeth.1277. [DOI] [PubMed] [Google Scholar]