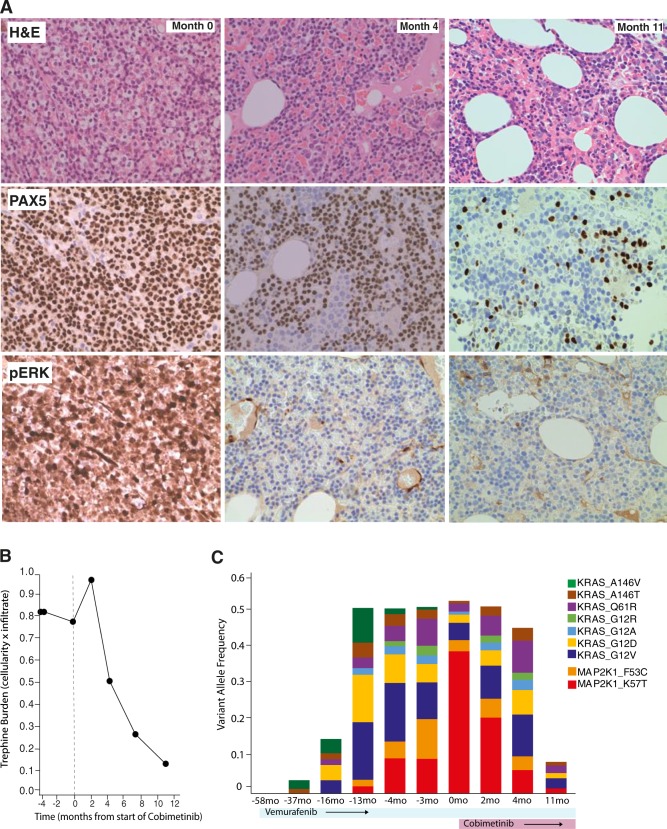
Fig. 2.
a Bone marrow trephine biopsies stained with H&E (top) or PAX5 antibody (middle) or pERK (lower) taken at the indicated time points relative to start of cobimetinib. b Leukemic burden prior to and after starting cobimetinib therapy was calculated as the product of bone marrow trephine cellularity and leukemic cell infiltrate. c Mutant allele frequency for the indicated KRAS and MAP2K1 mutations quantified by targeted amplicon sequencing at multiple time point relative to treatment