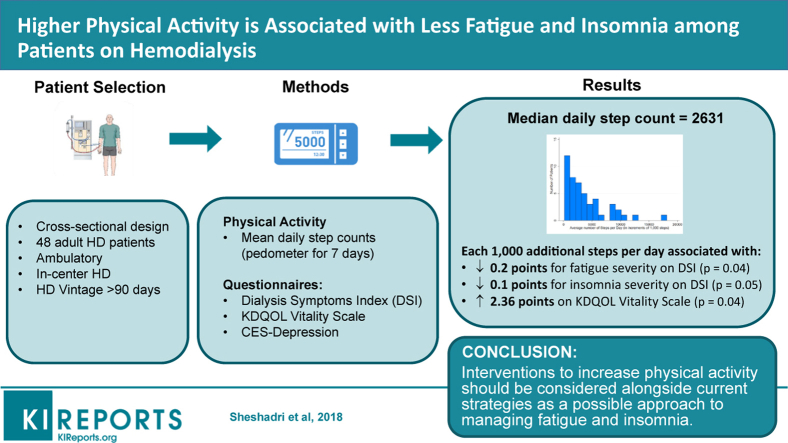
Abstract
Introduction
Patients on hemodialysis experience a heavy burden of symptoms that may be related to the low levels of physical activity reported in this population. We hypothesized that physical activity would be inversely related to symptom severity and that depression might mediate this association.
Methods
We designed a cross-sectional study of 48 patients receiving hemodialysis at 3 San Francisco dialysis clinics. Physical activity was measured using pedometers and recorded within 1 week of symptom assessment. Symptoms were assessed using total symptom burden and severity on the Dialysis Symptom Index (DSI; burden 0–29, severity 0–145), individual symptoms on the DSI (0–5), Kidney Disease Quality of Life Vitality scores, (0–100), and the Center for Epidemiologic Study-Depression (0–60).
Results
Median daily step count was 2631 (25th, 75th percentile 1125, 5278). Seventy-three percent of patients reported fatigue. After adjustment for age, sex, diabetes, and serum albumin, physical activity was associated with 0.2 points lower fatigue severity per 1000 steps per day (95% confidence interval [CI] −0.3 to 0.0), P = 0.04. Physical activity was also associated with higher Vitality score (2.36 points per 1000 steps; 95% CI 0.07–4.65) and lower insomnia scores (−0.1 points per 1000 steps; 95% CI −0.3 to 0.0], P < 0.05) in our adjusted models. Physical activity was not associated with other symptoms.
Conclusion
Because the study was cross-sectional, we cannot determine whether physical activity lowers fatigue and insomnia or whether less insomnia and fatigue increase physical activity. However, interventions to increase physical activity should be considered alongside current strategies as a possible approach to managing fatigue and insomnia.
Keywords: dialysis, fatigue, physical activity, QoL, symptoms
Graphical abstract
Patients on hemodialysis experience a heavy burden of symptoms in terms of number, frequency, and severity. Fatigue, weakness, muscle cramps, and difficulty sleeping are among the most prominently reported symptoms.1, 2, 3 In addition, depressive symptoms and anxiety are prevalent even in the absence of a formal diagnosis of depression.4, 5 Fatigue, in particular, has been singled out as the most common and bothersome symptom,1, 2 and many patients and caregivers ranked it as a more important consideration in their hemodialysis experience than survival.6, 7
It is unclear whether patients’ symptoms are related directly to end-stage renal disease, to the dialysis treatment, or to other factors, such as the very low levels of physical activity that are characteristic of the dialysis population.8, 9, 10 It may be that low levels of physical activity, independent of other dialysis-related factors, directly contribute to the fatigue, muscle soreness, cramping, and insomnia that many dialysis patients experience.
We therefore sought to ascertain whether physical activity was related to symptoms experienced by patients on hemodialysis. Given that physical inactivity is associated with depression in the general population,11, 12 and depression might also be related to the experience of other symptoms, we also aimed to evaluate the extent to which associations between physical activity and symptoms were independent of depression. We hypothesized that physical activity would be inversely related to symptom severity, particularly for fatigue. We further hypothesized that depression might partially mediate the association between physical activity and fatigue.
Methods
Study Participants
We enrolled hemodialysis patients from 3 San Francisco dialysis clinics. Inclusion criteria included age ≥18 years, receiving thrice-weekly in-center hemodialysis, being on dialysis for at least 90 days, having access to a telephone, and being ambulatory. Participants who used a cane or other assistive device for walking were included, but patients who used a wheelchair or scooter part-time were excluded. No patients had any amputations preventing walking. A total of 48 patients were included in the study. Patients were asked their race and ethnicity (Hispanic or non-Hispanic), and their medical records were reviewed to ascertain information about the dialysis prescription and laboratory results, comorbid conditions, and prescribed medications. Patients provided written informed consent to participate, and the study was approved by the University of California, San Francisco Committee on Human Research.
Physical Activity Measurement
Physical activity was measured using pedometers (AE120; Accusplit, Livermore, CA), which are an accurate and well-studied tool to measure walking.13, 14, 15, 16 Participants were instructed to wear the pedometer on their belt or waistband continuously during waking hours and to record daily steps during the week before or after testing. Step counts were recorded in a log each day and then the pedometer reset to obtain step counts for the next 24-hour period. Participants were instructed to record and reset the pedometer at the same time each day during the recording period. Pedometer placement was calibrated to individual participants before the participant took the pedometer home. They were asked to relay their step counts to study personnel in person at their regular dialysis session. The average daily step count over the week of recording was obtained from these step counts.
Symptom Assessment
Assessment was performed immediately on starting a mid-week hemodialysis session (Wednesday, Thursday, Friday, or Saturday). We used the modified DSI, as well as items from the Kidney Disease Quality of Life Vitality Scale. The modified DSI is a 29-item list of symptoms that was developed specifically for patients treated with dialysis. The questionnaire asks participants about the presence or absence of various symptoms within the past week and how bothersome the symptoms were on a scale from 1 (not at all) to 5 (very much). Scores on the DSI range from 0 (no symptoms at all) to 145 (all 29 symptoms rated as very much bothersome). There are 2 questions specifically related to fatigue and 2 questions specifically related to insomnia. The Kidney Disease Quality of Life Vitality score includes 4 items related to the frequency of fatigue-related experiences within the past month. The score ranges from 0 to 100, with a higher score indicating less fatigue.
To ascertain whether depression might mediate any association between physical activity and symptoms, we also administered the Center for Epidemiologic Studies Depression Scale (CES-D). The CES-D is a screening tool for depression that has 20 statements about emotions and asks how often these statements applied to a participant within the past week. Each item is scored from 0 to 3, and the total score ranges from 0 to 60.
Statistical Analysis
Patients’ characteristics, physical activity level, and questionnaire scores were summarized as median (25th, 75th percentile) for continuous variables or number and percentage for categorical variables. For physical activity, we calculated the mean daily steps for each participant and then reported the median (25th, 75th percentile) for the group. Univariable and multivariable linear regression analyses were used to examine associations between physical activity and symptoms. Linear regression analyses were also tested for heteroskedasticity using the Breusch-Pagan method. Multivariable analyses were adjusted for age, sex, diabetic status, and serum albumin (as a surrogate marker of nutritional status). These covariates were chosen based on prior literature10, 17, 18, 19, 20 and expert opinion. Additional sensitivity analysis was performed using linear regression and quintile of physical activity as predictor. We also planned to conduct formal mediation analyses to ascertain whether physical activity was associated with depression and whether depression mediated any associations between physical activity and symptoms. The threshold for statistical significance was P < 0.05. Statistical analyses were performed with Stata, version 14 (StataCorp, College Station, TX).
Results
Patient Characteristics, Activity, and Symptoms
A total of 101 potentially eligible patients were approached over the course of 20 months to reach the target of 48 participants. Demographic characteristics of those who refused to participate were similar to those enrolled in the study. Specifically, the median age of the nonparticipants was 60.5 years (25th, 75th percentile 50.5–69.0), and 71.7% were men. Nineteen percent of nonparticipants identified as of Hispanic or Latino ethnicity. Twenty-five percent were white, 30% black, 25% Asian, and the remainder, other. The median age of actual participants was 57 years. Eighty-one percent were men. Nineteen percent of participants identified as of Hispanic or Latino ethnicity. Eight percent reported white as their race, 46% black, 19% Asian, and 27% other (Table 1). Forty percent of participants had diabetes, and 94% had hypertension.
Table 1.
Patient characteristics at baseline for 48 hemodialysis patients
| Characteristic | n = 48 |
|---|---|
| Age, yr | 57 (52, 65) |
| Sex, % male | 81 |
| Hispanic, % | 19 |
| Race, % | |
| White | 8 |
| Black | 46 |
| Asian | 19 |
| Native Hawaiian/Pacific Islander | 8 |
| More than 1 race | 8 |
| Unknown/Unreported | 11 |
| Body mass index, kg/m2 | 29.5 (25.5, 34.6) |
| Comorbidities, % | |
| Hypertension | 94 |
| Diabetes mellitus | 40 |
| Coronary artery disease | 35 |
| Congestive heart failure | 31 |
| Stroke | 13 |
| Peripheral vascular disease | 10 |
| HIV | 2 |
| Arrhythmia | 17 |
| Dialysis vintage, yr | 3.1 (1.0, 6.3) |
| Hemoglobin, mg/dl | 10.8 (9.8, 11.8) |
| Albumin, mg/dl | 3.9 (3.8, 4.2) |
| Kt/V | 1.48 (1.34, 1.61) |
| Education, % | |
| High school or less | 44 |
| Vocational or some college | 31 |
| College degree | 15 |
| Professional or graduate degree | 10 |
| Currently smoking, % | 19 |
Values are reported as median (25th, 75th percentile).
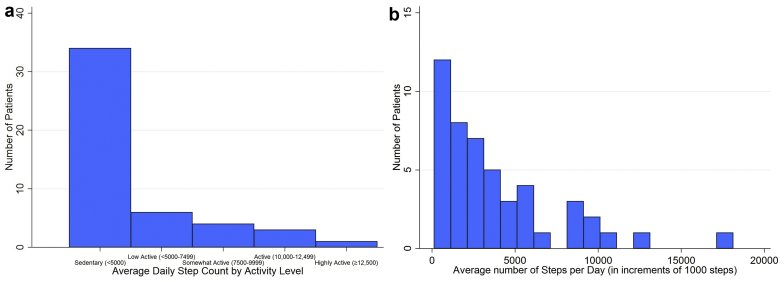
Patients’ median daily step count was 2631 (1125, 5278) (Table 2), and most patients’ mean step counts classified them as sedentary (Figure 1a and b). Ninety-eight percent had at least one symptom, and the median number of symptoms was 9 (6, 12). The median total symptom severity score on the DSI was 27 (14.0, 38.5). Seventy-three percent of patients reported fatigue, the most commonly reported and most bothersome symptom (median symptom severity score of 1.5 [0, 3.5]). The median CES-D score was 5.5 (2, 10), and 8 patients (16.7%) met the cutoff for depression.
Table 2.
Physical activity and questionnaire scores in 48 hemodialysis patients
| Activity or score | Median (25th, 75th percentile) |
|---|---|
| Average daily steps | 2631 (1125, 5278) |
| DSI total symptom burden | 9 (6, 12) |
| DSI total symptom severity | 27 (14, 38.5) |
| Individual symptoms on DSI, % | |
| (“feeling tired” or “having low energy”) | 73 |
| Muscle soreness | 21 |
| Muscle cramping | 48 |
| Insomnia (“trouble falling asleep” or “trouble staying asleep”) | 56 |
| Bone or joint pain | 44 |
| CES-D total score | 5.5 (2, 10) |
| KDQOL Vitality scorea | 50 (40, 80) |
CES-D, Centers for Epidemiologic Studies Depression Scale; DSI, Dialysis Symptom Index; KDQOL, Kidney Disease Quality of Life.
KDQOL Vitality Score from 47 patients who completed data
Figure 1.
(a) Distribution of daily step counts in 48 hemodialysis patients by activity level. (b) Distribution of daily step counts in 48 hemodialysis patients per 1000 steps.
Association Between Physical Activity and Symptoms
Physical activity was inversely associated with the severity of fatigue. In univariable analysis, patients reported that fatigue was 0.1 points less bothersome for every 1000 more steps per day (95% CI −0.3 to 0; P = 0.03; Table 3). Physical activity remained associated with fatigue after adjustment for age, sex, diabetes, and serum albumin (−0.2; 95% CI −0.3 to 0.0; P = 0.04). None of the covariates included in multivariable analyses were significantly associated with fatigue apart from serum albumin. Physical activity was also associated with the Kidney Disease Quality of Life Vitality score (2.53 points [95% CI 0.53–4.52] per 1000 steps per day in univariable analysis, and 2.36 [95% CI 0.07–4.65] in adjusted analysis). Only physical activity had a significant association with this measure of fatigue.
Table 3.
Association of average daily step count (per 1000 steps) with physical and emotional symptoms in 48 hemodialysis patients using linear regression analysis
| Symptom or score | Unadjusted |
Adjusteda |
||
|---|---|---|---|---|
| Coefficient (95% CI) | P | Coefficient (95% CI) | P | |
| DSI total symptom burden | 0.0 (−0.4 to 0.4) | >0.9 | 0.0 (−0.4 to 0.4) | 0.87 |
| DSI total symptom severity | −1.0 (−2.5 to 0.5) | 0.20 | −0.9 (−2.6 to 0.7) | 0.26 |
| DSI fatigue (“feeling tired” or “having low energy”) | −0.1 (−0.3 to 0.0) | 0.03 | −0.2 (−0.3 to 0.0) | 0.04 |
| DSI muscle soreness | −0.1 (−0.2 to 0) | 0.06 | −0.1 (−0.2 to 0.1) | 0.21 |
| DSI muscle cramping | 0.03 (−0.1 to 0.2) | 0.64 | 0.1 (−0.02, 0.3) | 0.10 |
| DSI insomnia (“trouble falling asleep” or “trouble staying asleep”) | −0.1 (−0.2 to 0.1) | 0.36 | −0.1 (−0.3 to 0.0) | 0.05 |
| DSI bone or joint pain | −0.04 (−0.2, 0.1) | 0.65 | −0.03 (−0.2 to 0.2) | 0.80 |
| KDQOL Vitality scoreb | 2.53 (0.53 to 4.52) | 0.01 | 2.36 (0.07 to 4.65) | 0.04 |
| CES-D total score | 0.1 (−0.7 to 0.9) | 0.84 | 0.0 (−1.0 to 0.9) | >0.9 |
CES-D, Centers for Epidemiologic Studies Depression Scale; DSI, Dialysis Symptom Index; KDQOL, Kidney Disease Quality of Life.
Adjustment for age, sex, diabetic status, serum albumin.
KDQOL Vitality score from 47 patients who completed data.
Patients who were more active also reported being less bothered by insomnia (trouble falling asleep or trouble staying asleep) in adjusted analysis (−0.1 points per 1000 steps per day; 95% CI −0.3 to 0.0; P < 0.05) but not in univariable analysis. Physical activity was not associated with the total DSI symptom burden or severity score or with individual symptoms of muscle soreness, bone or joint pain, or muscle cramping.
We did not find evidence of heteroskedasticity in any of our analyses.
Potential Mediation by Depression
We had hypothesized that physical activity would be associated with symptoms of depression and that depressive symptoms might partially mediate associations between physical activity and fatigue; however, physical activity was not associated with CES-D score (0.1 points per 1000 steps per day [95% CI −0.7 to 0.9]). We therefore did not perform formal mediation analysis of depression as a potential mediator of the association between physical activity and fatigue or other symptoms.
Sensitivity Analysis
Additional unadjusted sensitivity analysis using quintile of physical activity as a predictor showed a linear association of quintile of physical activity with lower fatigue score on DSI (−0.34 points on DSI per higher quintile of physical activity [95% CI −0.64 to −0.03] and 5.21 points higher on Kidney Disease Quality of Life Vitality score per higher quintile of physical activity [95% CI 0.33, 10.1]) but not with insomnia or other symptoms (Supplementary Table S1). After adjustment, quintile of physical activity remained associated with fatigue score on the DSI (−0.34; 95% CI −0.68 to 0.0; P < 0.05).
Discussion
Most patients in our study reported fewer than 5000 steps per day, indicating that they can be considered sedentary; only 3 patients were “active” and 1 patient “highly active.”14 Seventy-three percent of patients in our study reported feeling fatigue, and 56% reported symptoms of insomnia. We noted that patients who walked more reported less fatigue, and this finding was consistent whether they were asked to endorse statements about the feeling of fatigue on the Vitality scale or were asked how much they were bothered by fatigue on the DSI.
Our findings that hemodialysis patients are relatively sedentary13 and that a large percentage experienced fatigue and insomnia7, 21, 22, 23 are in agreement with other studies. Although one might assume that fatigue and insomnia would be associated with physical activity based on inferences from the general population, dialysis patients have a much higher prevalence of depression,5 inflammation,24 and comorbid conditions19 than the general population. These factors could subsume or mask any association of physical activity with symptoms, and indeed several other associations differ in dialysis patients from the general population (e.g., the obesity paradox).25 To our knowledge, our results are novel in that the association between objective measures of physical activity and fatigue has not been previously evaluated in this population. Gordon et al.26 reported that physcial activity assessed using the Human Activity Protocol was associated with postdialysis fatigue, but they did not examine general fatigue. Of note, fatigue was identified as a critically important consideration for patients treated with hemodialysis and providers of dialysis care in a recent report from the Standardised Outcomes in Nephrology initiative,6, 7 which sought to establish a consensus set of core outcomes for hemodialysis based on shared priorities of patients and caregivers.27
It is important to consider whether the association between physical activity and fatigue we observed is likely to be clinically meaningful. The minimum clinically important difference in the Vitality scale is considered to be 5 to 10 points.28 In our study, every 2000 steps was associated with 5 points higher on the Vitality scale, and 4000 steps was associated with nearly a 1-point change in symptom severity (e.g., from “not at all bothered” to “somewhat bothered” or “somewhat bothered” to “bothered quite a bit”). Although the minimum clinically important difference for this question has not been formally established, this difference would be expected to be meaningful for patients. These results suggest that differences in physical activity, even at the relatively low levels performed by these patients, are associated with clinically meaningful differences in patient-reported fatigue.
Although the association between physical activity and fatigue appears to be clinically significant, the reason for the association requires additional consideration. We hypothesize that physical activity reduces fatigue. Possible mechanisms might include an anti-inflammatory effect of exercise,29, 30 increased muscle strength or endurance,31, 32 prevention of loss of muscle mass,33 or overall improvement in quality of life.34, 35 However, our cross-sectional analysis does not rule out an association in the other direction. Fatigue has been reported as a common barrier to exercise and physical activity among patients treated with dialysis,18 so it is plausible that fatigue could lead patients to be less active.
We had initially hypothesized that depression may mediate an association between physical activity and fatigue or other symptoms. Prior studies have found an association between physical activity, either by self-report (using the Human Activity Protocol ) or based on objective accelerometer data, and depression as measured by the Beck’s Depression Index.36 However, we found no association between physical activity and depressive symptoms in univariable or multivariable analysis. The lack of association in our study may be because the overall level of depression in our population was relatively low. Only 8 (16.7%) of our participants met the standard cutoff for depression on the CES-D of a score of ≥16. Some researchers have suggested that given the burden of ESRD, a more accurate cutoff would be ≥18,37 which only 4 (8.3%) of our patients met.
We also hypothesized that changes in step count would be associated with changes in the insomnia score. Many studies in the general population have focused on at least moderate aerobic exercise as a means of alleviating symptoms of insomnia or improving health.38, 39, 40 For example, in one systematic review of the effects of exercise on insomnia in middle-aged women, programmed aerobic exercise improved sleep quality, but low levels of physical activity had no statistically significant effect.41 However, in the relatively sedentary dialysis population, both a prior study by Anand et al.,42 and now our study, show that differences in physical activity are associated with fewer self-reported sleep symptoms despite low overall levels of activity. Of note, the DSI includes only 2 questions about sleep and specifically does not address early-morning awakening, which had the strongest association with physical activity in the prior study.42
Although many of our patients reported muscle cramping, muscle soreness, and bone and joint pain, physical activity was not significantly associated with these symptoms. We had hypothesized that more active patients might report less musculoskeletal pain because exercise is recommended for alleviation of knee arthritis43 even in patients with baseline physical dysfunction44 and is also thought to be of benefit in rheumatoid arthritis.45 However, pain has also been reported as a barrier to activity and exercise by patients treated with dialysis.18 In this light, it may be that the DSI is not a sensitive enough instrument to detect differences in individual pain-related symptoms that may be important. The low intensity of activity could also be a reason that step counts were not associated with some symptoms. Higher-intensity activity or greater amounts of low-intensity activity may be needed to address pain. Finally, there are multiple mechanisms for pain in the dialysis population,46 and it may be that physical activity does not address the causes of pain experienced by patients on dialysis.
Our study has several limitations that should be acknowledged. Because the study is cross-sectional, we cannot determine if physical activity lowers the burden of fatigue, if fatigue leads to lower physical activity, or whether they are associated through another mechanism (although depression did not appear to mediate). The study participants were also selected entirely from study centers in Northern California, which may limit generalizability of the number of step counts reported to the broader US and international dialysis populations; for example, European dialysis patients have a higher baseline physical activity than patients in the United States.15 However, we do not think that this should affect the association between physical activity and symptoms. Our sample size was relatively small, which limited the number of variables we could include in the adjusted analysis. We were not able to consider all possible mediators or colliders (e.g., body mass index) in our model.
Patients participating in the study were all given pedometers, which itself could have motivated them to increase activity.16 However, if this were true, it would mask even lower levels of activity and would likely bias associations toward the null rather than cause a spurious association with fatigue. Because of how the pedometer recordings were logged, we do not believe there was any recall bias. We acknowledge that there may have been willful misreporting, but we did not see any evidence to support that possibility. Step counts measure only walking, which does not include the full spectrum of physical activity. However, walking is the most common form of physical activity for most patients treated with dialysis.47 Pedometers are unable to capture information that more modern activity trackers can record, such as intensity of activity or duration of individual episodes of activity. This type of data would have further informed our interpretation of the results. We also recognize that patients who are unable to walk and were excluded from the study could have a different association of physical activity with symptom burden or severity. The instruments available to assess symptoms among patients on dialysis are geared toward total symptom burden rather than in-depth examination of key symptoms. More detailed measures of symptoms in this population may be needed to fully evaluate their association with physical activity.
Most patients in our cohort were sedentary; however, variations in activity level even at this low end of the spectrum were associated with fatigue and insomnia, which are important, pervasive, and difficult to manage in patients treated with dialysis. Interventions to increase physical activity should be considered to help manage fatigue and insomnia, either through counseling or more directed programs.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Dr. Sheshadri’s effort was supported by an American Kidney Fund Clinical Scientist in Nephrology Fellowship and a Ruth L. Kirschstein National Research Service Award Individual Postdoctoral Fellowship (F32 DK111154-02). ClinicalTrials.gov ID NCT02623348. Dr. Kittiskulnam received support from an International Society of Nephrology fellowship. Dr. Johansen’s effort was supported by a Midcareer Investigator Award in Patient Oriented Research (K24-DK085153).
Contributions: Research idea and study design: AS, PK, KJ; data acquisition: AS, PK; statistical analysis and interpretation: AS; supervision and mentorship: KJ. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Footnotes
Table S1. Association of average daily step count per quintile of physical activity with physical and emotional symptoms in 48 hemodialysis patients using linear regression analysis.
Supplementary material is linked to the online version of the paper at http://www.kireports.org/.
Supplementary Material
Association of average daily step count per quintile of physical activity with physical and emotional symptoms in 48 hemodialysis patients using linear regression analysis.
References
- 1.Abdel-Kader K., Unruh M.L., Weisbord S.D. Symptom burden, depression, and quality of life in chronic and end-stage kidney disease. Clin J Am Soc Nephrol. 2009;4:1057–1064. doi: 10.2215/CJN.00430109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zukiman W.Z.H.W., Yaakup H., Zakaria N.F. Symptom prevalence and the negative emotional states in end-stage renal disease patients with or without renal replacement therapy: a cross-sectional analysis. J Palliat Med. 2017;20:1127–1134. doi: 10.1089/jpm.2016.0450. [DOI] [PubMed] [Google Scholar]
- 3.Weisbord S.D., Fried L.F., Arnold R.M. Prevalence, severity, and importance of physical and emotional symptoms in chronic hemodialysis patients. J Am Soc Nephrol. 2005;16:2487–2494. doi: 10.1681/ASN.2005020157. [DOI] [PubMed] [Google Scholar]
- 4.Kimmel P. Psychosocial factors in dialysis patients. Kidney Int. 2001;59:1599–1613. doi: 10.1046/j.1523-1755.2001.0590041599.x. [DOI] [PubMed] [Google Scholar]
- 5.Alavi N.M., Aliakbarzadeh Z., Sharifi K. Depression, anxiety, activities of daily living, and quality of life scores in patients undergoing renal replacement therapies. Transplant Proc. 2009;41:3693–3696. doi: 10.1016/j.transproceed.2009.06.217. [DOI] [PubMed] [Google Scholar]
- 6.Urquhart-Secord R., Craig J.C., Hemmelgarn B. Patient and caregiver priorities for outcomes in hemodialysis: an International Nominal Group Technique Study. Am J Kidney Dis. 2016;68:444–454. doi: 10.1053/j.ajkd.2016.02.037. [DOI] [PubMed] [Google Scholar]
- 7.Evangelidis N., Tong A., Manns B. Developing a set of core outcomes for trials in hemodialysis: an International Delphi Survey. Am J Kidney Dis. 2017;70:464–475. doi: 10.1053/j.ajkd.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 8.Johansen K.L., Painter P., Delgado C. Characterization of physical activity and sitting time among patients on hemodialysis using a new physical activity instrument. J Ren Nutr. 2015;25:25–30. doi: 10.1053/j.jrn.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johansen K.L., Chertow G.M., Kutner N.G. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78:1164–1170. doi: 10.1038/ki.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johansen K.L., Chertow G.M., Ng A.V. Physical activity levels in patients on hemodialysis and healthy sedentary controls. Kidney Int. 2000;57:2564–2570. doi: 10.1046/j.1523-1755.2000.00116.x. [DOI] [PubMed] [Google Scholar]
- 11.Stephens T Physical activity and mental health in the United States and Canada: evidence from four population surveys. Prev Med. 1988;17:35–47. doi: 10.1016/0091-7435(88)90070-9. [DOI] [PubMed] [Google Scholar]
- 12.Dunn A.L., Trivedi M.H., O'Neal H.A. Physical activity dose-response effects on outcomes of depression and anxiety. Med Sci Sports Exerc. 2001;33:S587–S597. doi: 10.1097/00005768-200106001-00027. 609–510. [DOI] [PubMed] [Google Scholar]
- 13.Tudor-Locke C., Washington T.L., Hart T.L. Expected values for steps/day in special populations. Prev Med. 2009;49:3–11. doi: 10.1016/j.ypmed.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Tudor-Locke C., Bassett D.R., Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 15.Nowicki M., Murlikiewicz K., Jagodzińska M. Pedometers as a means to increase spontaneous physical activity in chronic hemodialysis patients. J Nephrol. 2010;23:297–305. [PubMed] [Google Scholar]
- 16.Bravata D.M., Smith-Spangler C., Sundaram V. Using pedometers to increase physical activity and health: a systematic review. JAMA. 2007;298:2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 17.Johansen K.L., Kaysen G.A., Dalrymple L.S. Association of physical activity with survival among ambulatory patients on dialysis: the Comprehensive Dialysis Study. Clin J Am Soc Nephrol. 2013;8:248–253. doi: 10.2215/CJN.08560812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Delgado C., Johansen K.L. Barriers to exercise participation among dialysis patients. Nephrol Dial Transplant. 2012;27:1152–1157. doi: 10.1093/ndt/gfr404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kittiskulnam P., Sheshadri A., Johansen K.L. Consequences of CKD on functioning. Semin Nephrol. 2016;36:305–318. doi: 10.1016/j.semnephrol.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johansen K.L. Exercise in the end-stage renal disease population. J Am Soc Nephrol. 2007;18:1845–1854. doi: 10.1681/ASN.2007010009. [DOI] [PubMed] [Google Scholar]
- 21.Hamzi M.A., Hassani K., Asseraji M. Insomnia in hemodialysis patients: a multicenter study from Morocco. Saudi J Kidney Dis Transpl. 2017;28:1112–1118. doi: 10.4103/1319-2442.215152. [DOI] [PubMed] [Google Scholar]
- 22.Liaveri P.G., Dikeos D., Ilias I. Quality of sleep in renal transplant recipients and patients on hemodialysis. J Psychosom Res. 2017;93:96–101. doi: 10.1016/j.jpsychores.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 23.Jhamb M., Weisbord S.D., Steel J.L. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am J Kidney Dis. 2008;52:353–365. doi: 10.1053/j.ajkd.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Himmelfarb J., Stenvinkel P., Ikizler T.A. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62:1524–1538. doi: 10.1046/j.1523-1755.2002.00600.x. [DOI] [PubMed] [Google Scholar]
- 25.Kalantar-Zadeh K., Kopple J.D. Obesity paradox in patients on maintenance dialysis. Contrib Nephrol. 2006;151:57–69. doi: 10.1159/000095319. [DOI] [PubMed] [Google Scholar]
- 26.Gordon P.L., Doyle J.W., Johansen K.L. Postdialysis fatigue is associated with sedentary behavior. Clin Nephrol. 2011;75:426–433. [PubMed] [Google Scholar]
- 27.Tong A., Manns B., Hemmelgarn B. Establishing core outcome domains in hemodialysis: report of the Standardized Outcomes in Nephrology-Hemodialysis (SONG-HD) Consensus Workshop. Am J Kidney Dis. 2017;69:97–107. doi: 10.1053/j.ajkd.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bjorner J.B., Wallenstein G.V., Martin M.C. Interpreting score differences in the SF-36 Vitality scale:using clinical conditions and functional outcomes to define the minimally important difference. Curr Med Res Opin. 2007;23:731–739. doi: 10.1185/030079907x178757. [DOI] [PubMed] [Google Scholar]
- 29.Nimmo M.A., Leggate M., Viana J.L. The effect of physical activity on mediators of inflammation. Diabetes Obes Metab. 2013;14:51–60. doi: 10.1111/dom.12156. [DOI] [PubMed] [Google Scholar]
- 30.Loprinzi P.D. Frequency of moderate-to-vigorous physical activity (MVPA) is a greater predictor of systemic inflammation than total weekly volume of MVPA: implications for physical activity promotion. Physiol Behav. 2015;141:46–50. doi: 10.1016/j.physbeh.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Morishita Y., Kubo K., Miki A. Positive association of vigorous and moderate physical activity volumes with skeletal muscle mass but not bone density or metabolism markers in hemodialysis patients. Int Urol Nephrol. 2014;46:633–639. doi: 10.1007/s11255-014-0662-9. [DOI] [PubMed] [Google Scholar]
- 32.Morishita Y., Nagata D. Strategies to improve physical activity by exercise training in patients with chronic kidney disease. Int J Nephrol Renovasc Dis. 2015;8:19–24. doi: 10.2147/IJNRD.S65702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kouidi E.J., Grekas D.M., Deligiannis A.P. Effects of exercise training on noninvasive cardiac measures in patients undergoing long-term hemodialysis: a randomized controlled trial. Am J Kidney Dis. 2009;54:511–521. doi: 10.1053/j.ajkd.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Tentori F., Elder S.J., Thumma J. Physical exercise among participants in the Dialysis Outcomes and Practice Patterns Study (DOPPS): correlates and associated outcomes. Nephrol Dial Transplant. 2010;25:3050–3062. doi: 10.1093/ndt/gfq138. [DOI] [PubMed] [Google Scholar]
- 35.Kouidi E. Health-related quality of life in end-stage renal disease patients: the effects of renal rehabilitation. Clin Nephrol. 2004;61(Suppl 1):S60–S71. [PubMed] [Google Scholar]
- 36.Zhang M., Kim J.C., Li Y. Relation between anxiety, depression, and physical activity and performance in maintenance hemodialysis patients. J Ren Nutr. 2014;24:252–260. doi: 10.1053/j.jrn.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedayati S.S., Bosworth H.B., Kuchibhatla M. The predictive value of self-report scales compared with physician diagnosis of depression in hemodialysis patients. Kidney Int. 2006;69:1662–1668. doi: 10.1038/sj.ki.5000308. [DOI] [PubMed] [Google Scholar]
- 38.Sherrill D.L., Kotchou K., Quan S.F. Association of physical activity and human sleep disorders. Arch Intern Med. 1998;158:1894–1898. doi: 10.1001/archinte.158.17.1894. [DOI] [PubMed] [Google Scholar]
- 39.King A.C., Oman R.F., Brassington G.S. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. JAMA. 1997;277:32–37. [PubMed] [Google Scholar]
- 40.Riemann D., Baglioni C., Bassetti C. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26 doi: 10.1111/jsr.12594. 875–700. [DOI] [PubMed] [Google Scholar]
- 41.Rubio-Arias J.A., Marin-Cascales E., Ramos-Campo D.J. Effect of exercise on sleep quality and insomnia in middle-aged women: a systematic review and meta-analysis of randomized controlled trials. Maturitas. 2017;100:49–56. doi: 10.1016/j.maturitas.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Anand S., Johansen K.L., Dalrymple L.S. Physical activity and self-reported symptoms of insomnia, restless legs syndrome, and depression: the comprehensive dialysis study. Hemodial Int. 2013;17:50–58. doi: 10.1111/j.1542-4758.2012.00726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dunlop D.D., Song J., Semanik P.A. Physical activity levels and functional performance in the osteoarthritis initiative: a graded relationship. Arthritis Rheum. 2011;63:127–136. doi: 10.1002/art.27760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall M., Hinman R.S., van der Esch M. Is the relationship between increased knee muscle strength and improved physical function following exercise dependent on baseline physical function status? Arthritis Res Ther. 2017;19:271. doi: 10.1186/s13075-017-1477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veldhuijzen van Zanten J.J., Rouse P.C., Hale E.D. Perceived barriers, facilitators and benefits for regular physical activity and exercise in patients with rheumatoid arthritis: a review of the literature. Sports Med. 2015;45:1401–1412. doi: 10.1007/s40279-015-0363-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pham P.C., Khaing K., Sievers T.M. 2017 update on pain management in patients with chronic kidney disease. Clin Kidney J. 2017;10:688–697. doi: 10.1093/ckj/sfx080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allen K., Gappmaier E. Exercise habits and attitudes of patients undergoing hemodialysis. Cardiopulm Phys Ther J. 2001;12:11–16. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Association of average daily step count per quintile of physical activity with physical and emotional symptoms in 48 hemodialysis patients using linear regression analysis.