Abstract
The fish heart ventricle has varied morphology and may have a specific morpho‐functional design in species adapted to extreme environmental conditions. In general, the Amazonian ichthyofauna undergoes constant variations in water temperature, pH and oxygen saturation, which makes these species useful for investigations of cardiac morphology. Arapaima gigas, a member of the ancient teleost group Osteoglossomorpha, is one of the largest freshwater fish in the world. This species has a specific heart metabolism that uses fat as the main fuel when O2 supplies are abundant but also can change to glycogen fermentation when O2 content is limiting. However, no information is available regarding its heart morphology. Here, we describe the heart of A. gigas, with emphasis on the ventricular anatomy and myoarchitecture. Specimens of A. gigas weighing between 0.3 and 4040 g were grouped into three developmental stages. The hearts were collected and the anatomy analyzed with a stereomicroscope, ultrastructure with a scanning electron microscope, and histology using toluidine blue, Masson's trichrome and Sirius red stains. The ventricle undergoes morphological changes throughout its development, from the initial saccular shape with a fully trabeculated myocardium and coronary vessel restricted to the subepicardium (Type I) (group 1) to a pyramidal shape with mixed myocardium and coronary vessels that penetrate only to the level of the compact layer (Type II) (groups 2 and 3). The trabeculated myocardium has a distinct net‐like organization in all the specimens, differing from that described for other teleosts. This arrangement delimits lacunae with a similar shape and distribution, which seems to allow a more uniform blood distribution through this myocardial layer.
Keywords: morphology, myocardium, ultrastructure, ventricle
Introduction
The teleost heart is composed of six distinct anatomical components: the sinus venosus, atrium, atrioventricular (AV) segment, ventricle, conus arteriosus and bulbus arteriosus. The AV segment and the conus are short in length and are functionally better defined as connecting segments (Icardo, 2017). The ventricle varies in its morphology among species, with respect to the external shape, the organization of the myocardium and the arrangement of the coronary vessels (Santer et al. 1983; Tota et al. 1983; Farrell & Jones, 1992; Icardo, 2017). The external ventricular shape may vary from pyramidal to saccular or tubular (Santer et al. 1983; Santer, 1985) and the myocardium may be entirely trabeculated, composed only of a complex arrangement of trabecular sheets (i.e. spongy layer); or mixed, composed of an inner trabeculated myocardium that is surrounded by an outer compact layer of muscle bundles (Satchell, 1970; Santer & Greer Walker, 1980; Sánchez‐Quintana et al. 1996). The myocarium arrangement associated with the distribution of the coronary vessels. constitutes one of the most important ways to classify the fish heart ventricle, into four main types (I–IV) (Tota et al. 1983; Tota, 1989).
In addition to morphological variability, some species may have a specific morpho‐functional design as a form of adaptation to environments with extreme climate conditions (Tota et al. 1997). In the Amazon region, water bodies undergo seasonal changes in dissolved‐oxygen and carbon‐dioxide concentrations, with short‐term conditions of hypoxia or even anoxia (Val et al. 1998). According to Hochachka & Randall (1978), fish in the Amazon must deal with variations in oxygen content and pH, daily oscillations in temperature, extremely low ion content and seasonal dry periods.
Among the Amazonian teleosts, Arapaima gigas (Schinz, 1822), popularly known as pirarucu, is an obligatory air‐breather and one of the largest freshwater fish in the world (Val & Almeida‐Val, 1995). This species was originally included in the family Osteoglossidae together with Osteoglossum bicirrhosum but is currently classified in the Arapaimidae along with the African bonytongue Heterotis niloticus, in the ancient teleost group of the Osteoglossomorpha (Val & Almeida‐Val, 1995). Hochachka et al. (1978) showed that the myocardium has cardiomyocytes rich in mitochondria with specialized functions, abundant intracellular triglyceride and glycogen. The mitochondria are active in oxidative metabolism; the enzymology showed that the myocardium can oscillate between glycogen fermentation in limiting oxygen levels and fat catabolism when oxygen supplies are abundant. Hochachka et al. (1978) noted that, considering the specialized functions of the mitochondria, the pirarucu inner myocardium resembles the mammalian heart more closely than the typical teleost heart. However, no published information is available regarding the heart morphology of this species. Here, we describe the heart of A. gigas with emphasis on the ventricular anatomy and myoarchitecture.
Materials and methods
Animals
Thirty‐six specimens of Arapaima gigas weighing between 0.3 and 4040 g were obtained from fish farms in the states of Tocantins and Amapá, Brazil. The specimens were grouped into three stages of development for a more accurate morphological description of the ventricle and the myoarchitecture as the fish grows: Group 1 (0.3–4 g), Group 2 (327–691 g) and Group 3 (1360–4040 g). The fish were anesthetized and euthanized in saturated benzocaine hydrochloride solution, as approved by the Ethics Committee on Animal Use of the Federal University of Tocantins, Araguaína Campus (Proc. No. 231001.000872/2014‐49). The hearts were removed through a longitudinal incision on the anterior midventral line and then separated for anatomical, histological and ultrastructural studies.
Gross anatomy
For anatomical studies, three hearts from fish of each group (n = 9) were fixed in Bouin's solution (70% saturated picric acid solution, 25% formaldehyde and 5% acetic acid) for 24 h, stored in 70% ethanol and subsequently dissected for general analysis of the cardiac components. The components were documented with a Leica® camera (IC80HD) coupled to a Leica stereomicroscope (M50).
Conventional histology
For conventional histology studies, five hearts from fish of each group (n = 15) were used. The hearts were pre‐fixed in Bouin's solution, dehydrated in a graduated ethanol series and embedded in Paraplast® (McCormick Scientific®). Tissue sections 5–10 μm thick were cut and stained with Masson's trichrome (Bancroft & Layton, 2013) and Sirius red (Junqueira et al. 1979) for general analyses of the myoarchitecture and collagen‐fiber distribution.
Semithin sections
For precise analyses of the cardiac‐fiber organization, three hearts from fish of each group (n = 9) were used. Tissue fragments from the ventricle (groups 2 and 3) or the entire heart (group 1) were fixed in modified Karnovsky's solution (4% paraformaldehyde and 2.5% glutaraldehyde in phosphate buffer, pH 7.4) for 48 h, dehydrated in a graduated ethanol series and embedded in Historesin™ (Leica). Semithin 1‐μm‐thick sections were cut with a Leica microtome (RM2265) equipped with a glass blade. The sections were stained with toluidine blue with sodium tetraborate added for better contrast. The preparations were documented with a Leica camera (CC50HD) coupled to a Leica photomicroscope (DM750).
Scanning electron microscopy
Samples of three ventricles belonging to fish of group 3 (n = 3) were fixed in modified Karnovsky's solution for 48 h and post‐fixed in a buffer solution of 1% osmium tetroxide (pH 7.4) for 2 h. After dehydration in a graduated acetone series, they were processed in an EMS 850 Critical Point Dryer (Electron Microscopy Sciences), mounted on metal stubs and metallized with a colloidal gold‐palladium blend in a DESK II sputter coater (Denton Vacuum®). Analysis and documentation of the samples were carried out with a Zeiss® EVO MA10 scanning electron microscope.
Results
The heart of A. gigas is composed of six anatomical components arranged in a series: sinus venosus, atrium, atrioventricular segment, ventricle, conus arteriosus and bulbus arteriosus (Figs 1A–F and 2A–C). The sinus venosus, situated in the dorsal region of the atrium, has thin walls (Fig. 1D). The atrium, dorsal to the ventricle, has an irregular shape that varied slightly among the specimens (Fig. 1A–C). The atrium contacts the outflow tract laterally, due to the proximity of the inflow and outflow ventricular orifices (Figs 1A–C and 2A,B). Internally there is a series of thin trabeculae that constitutes the atrial myocardium (Fig. 1E,F). The subepicardial region is composed mainly of collagen, which is also present internally, involving the trabeculae (Fig. 1F).
Figure 1.
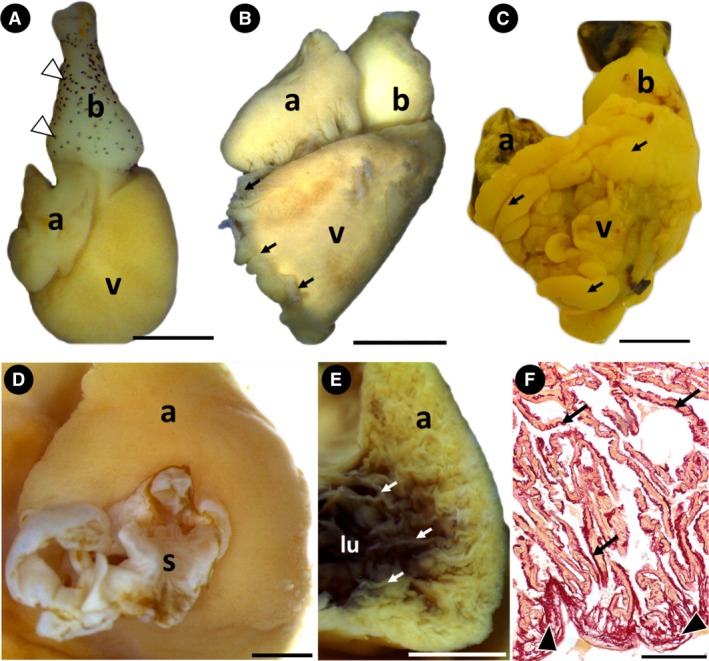
The Arapaima gigas heart. (A) Group 1. Heart showing atrium, ventricle and bulbus arteriosus. Note the saccular ventricle, the pyriform bulbus arteriosus and the pigmentation on its surface (white arrowheads) (lateral view). (B and C) Groups 2 and 3, respectively. In these specimens, the ventricle is pyramidal with adipose tissue visible on its surface (black arrows). The pigmentation is no longer visible on the surface of the bulbus arteriosus (lateral view). (D) Group 3. Sinus venosus and atrium (dorsal view). (E,F) Group 2. (E) Cross‐section of the atrium, showing the myocardium composed of thin trabeculae (white arrows). (F) Histological section showing the atrial subepicardium rich in collagen (black arrowheads) and atrial trabeculae involved by collagen fibers (black arrows). a, atrium; b, bulbus arteriosus; lu, lumen; s, sinus venosus; v, ventricle. Stain: F: Sirius red. Scale bars: (A) 1 mm; (B) 5 mm; (C) 1 mm; (D) 2 mm; (E) 2 mm; (F) 200 μm.
Figure 2.
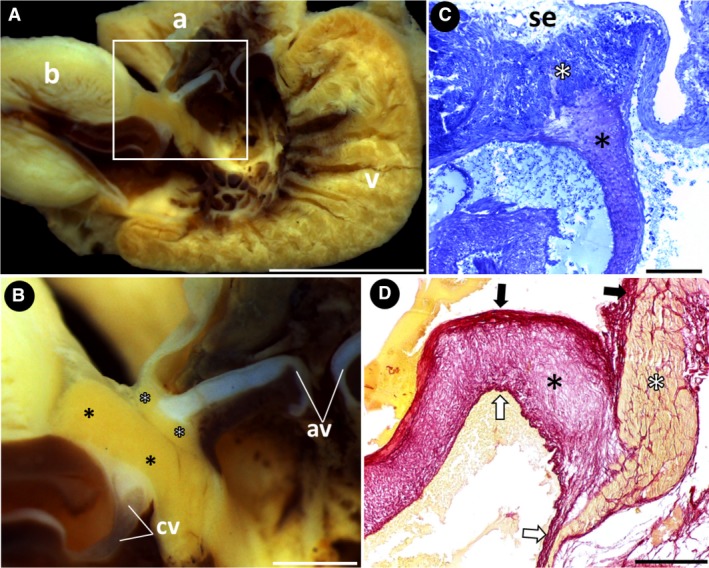
Heart structures and connecting segments. (A) Group 2. Sagittal section of the heart, showing the connecting segments (square). (B) Group 2. Detail of the connecting segments: the atrioventricular valves and conus valves are anchored to the atrioventricular segment (white asterisks) and the conus arteriosus (black asterisks), respectively. (C and D) Groups 1 and 2, respectively. Histological section of the atrioventricular valves (black asterisks) anchored to the atrioventricular segment (white asterisks), which is covered with subepicardial fibrous connective tissue. The valves have a dense core with a thick fibrous, collagen‐rich connective tissue, facing the atrial lumen (black arrows) and a thin fibrous connective tissue, facing the ventricular lumen (white arrows). a, atrium; av, atrioventricular valves; b, bulbus arteriosus; cv, conus valves; se, subepicardium; v, ventricle. Stains: (C) toluidine blue; (D) Sirius red. Scale bars: (A) 5 mm; (B) 1 mm; (C) 100 μm; (D) 200 μm.
In the transition region between the atrium and ventricle, the atrioventricular segment has a pair of valves (Fig. 2B,C). This segment consists of a compact vascularized myocardium surrounded by subepicardial fibrous connective tissue that extends into its musculature (Fig. 2C). Anchored in the segment musculature by connective tissue, the atrioventricular valves consist of a robust proximal base and a thinner distal portion that bends inside the ventricle. Three layers of connective tissue compose both portions: a dense core of loose connective tissue that has an extracellular matrix with sparsely organized collagen fibers, a thick inner lining of fibrous connective tissue with well‐oriented collagen fibers facing the atrium and a thin outer lining of fibrous connective tissue facing the ventricle (Fig. 2C,D).
The A. gigas ventricle undergoes anatomical changes throughout its development. In low‐weight specimens of group 1, the ventricle was saccular with rounded edges (Fig. 1A); in the other groups, it had a typical pyramidal shape with angular edges and a well‐defined apex (Fig. 1B,C). Also, the ventricular surface showed different proportions of adipose tissue, which was more abundant in high‐weight specimens of groups 2 and 3 (Fig. 1B,C).
The macroscopic and ultrastructural analyses of the inner surface of the ventricle revealed a group of trabecular sheets that radiate from the central lumen, delimiting a series of lacunae (i.e. intertrabecular spaces) that deepen toward the ventricular periphery (Fig. 3A–C). The organization pattern of the trabecular sheets and the similar distribution of the lacunae give the inner surface of the ventricle a net‐like appearance (Figs 3B and 4A). Within the lacunae, small openings between the trabecular sheets are evident, constituting an extensive system of microlacunae that allows communication among individual trabeculae (Fig. 4B–D). However, with histological studies, it was not possible to detect the microlacunae among the trabecular sheets in low‐weight specimens of group 1 (Fig. 5A,B). This region of the ventricular myocardium was characterized as the spongy layer and maintained the net‐like appearance in all the groups (Fig. 5A–D).
Figure 3.
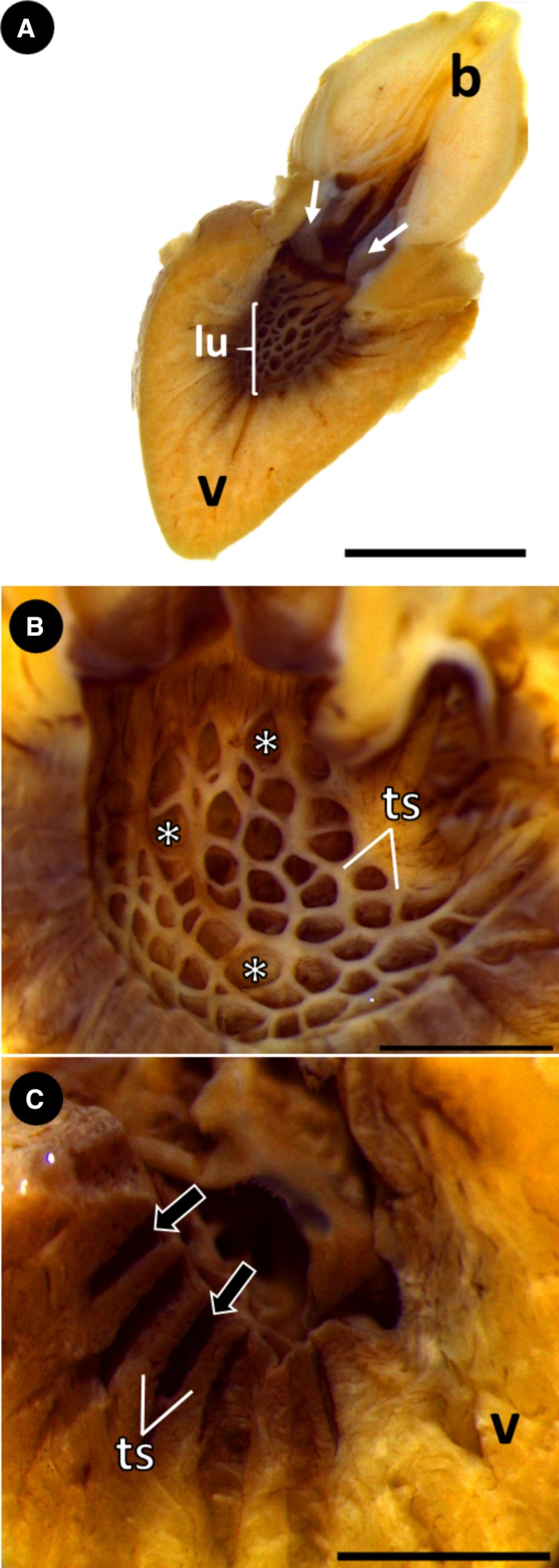
Ventricular structure of Group 2. (A) Sagittal section of the heart, showing the ventricular lumen, conus valves (white arrows) and bulbus arteriosus. (B) Sagittal section. Trabecular sheets in the myocardium, delimiting several lacunae (intertrabecular spaces) (white asterisks). Note the shape and distribution of the lacunae, lending the myocardium a net‐like appearance. (C) Sagittal section with detail of the lacunae (black arrows) deepening towards the ventricular periphery. b, bulbus arteriosus; lu, lumen; ts, trabecular sheets; v, ventricle. Scale bars: (A) 5 mm; (B) 2 mm; (C) 2 mm.
Figure 4.
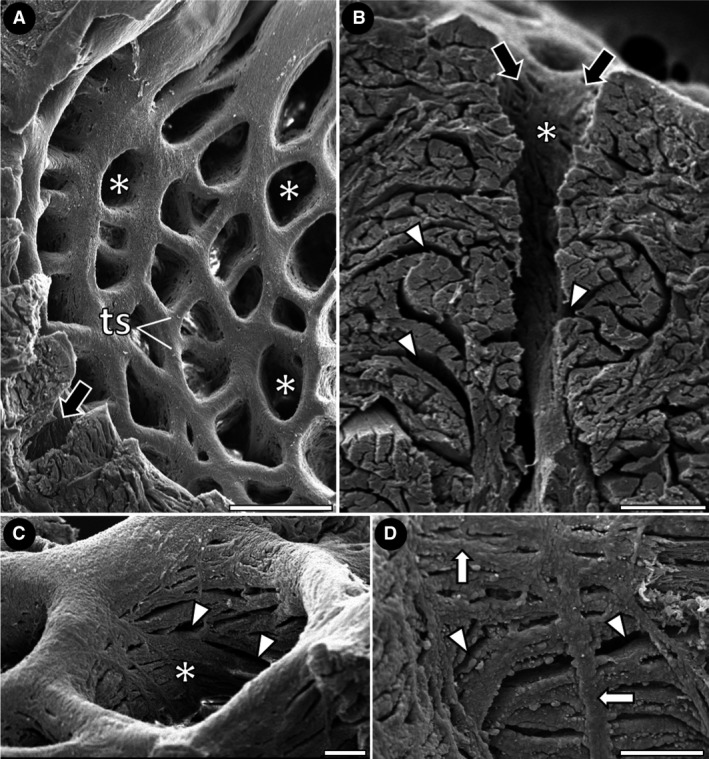
Scanning electron micrographs of the net‐like myocardium in Group 3. (A) Trabecular sheets delimiting lacunae with a similar shape and distribution (white asterisks). Note the extent of the lacunae towards the ventricular periphery (black arrow). (B‐D) Interior of lacunae, showing the opening of microlacunae (small openings among the trabecular sheets) (arrowheads). Note in D the trabecular sheets running in many directions (white arrows). ts, trabecular sheets. Scale bars: (A) 500 μm; (B) 100 μm; (C) 100 μm; (D) 100 μm.
Figure 5.
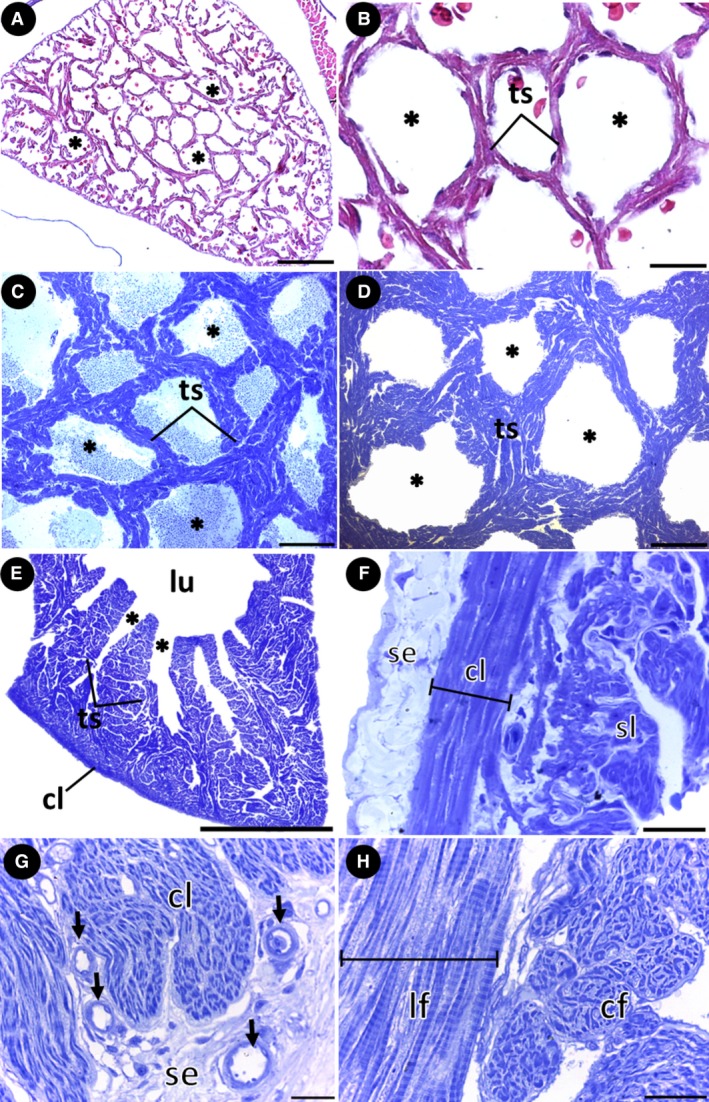
Myoarchitecture of the ventricle. (A,B) Cross‐section showing the net‐like arrangement in the trabeculated myocardium of low‐weight specimens of group 1. (C,D) Cross‐section showing the net‐like arrangement in the trabeculated myocardium of specimens of groups 2 and 3, respectively. (E) Sagittal histological section showing the lacunae opening from the lumen and the microlacunae connecting the individual trabeculae. (F) Organization of the outer compact layer under the subepicardium, involving the inner spongy layer. Note the cardiomyocytes arranged longitudinally. (G,H) Group 3. (G) Sagittal histological section showing coronary vessels in the subepicardium, penetrating the outer compact layer of the myocardium. Note the cardiomyocytes arranged circumferentially in the compact layer. (H) Cardiomyocytes arranged longitudinally and circumferentially in the spongy layer. *, lacunae; cf, circular fibers; cl, compact layer; lf, longitudinal fibers; lu, ventricular lumen; se, subepicardium; sl, spongy layer; ts, trabecular sheets. Stains: (A,B) Masson's trichrome; (C–H) Toluidine blue. Scale bars: (A) 100 μm; (B) 20 μm; (C) 200 μm; (D) 500 μm; (E) 2 mm; (F) 20 μm; (G) 20 μm; (H) 20 μm.
The myocardium, constituted entirely of a spongy layer, was observed only in specimens of group 1 (Fig. 5A,B). As the fish develop, a compact layer forms in the periphery of the myocardium, characterized by the progressive organization of cardiomyocytes into muscle bundles (groups 2 and 3) (Fig. 5E–G). The semithin longitudinal sections showed that the cardiomyocytes of the compact layer are oriented longitudinally and circumferentially (Fig. 5F,G). The cardiomyocytes of the spongy layer are similarly orientated (Fig. 5H). In the boundary between the two myocardial layers, a connective‐tissue rim enriched with collagen is present (Fig. 6A,B). Coiled collagen fibers are also evident in the connective tissue of the subepicardial region (Fig. 6A,B), involving the muscle bundles of the compact layer (Fig. 6A), the trabecular sheets of the spongy layer (Fig. 6C) and in the subendocardium (Fig. 6D).
Figure 6.
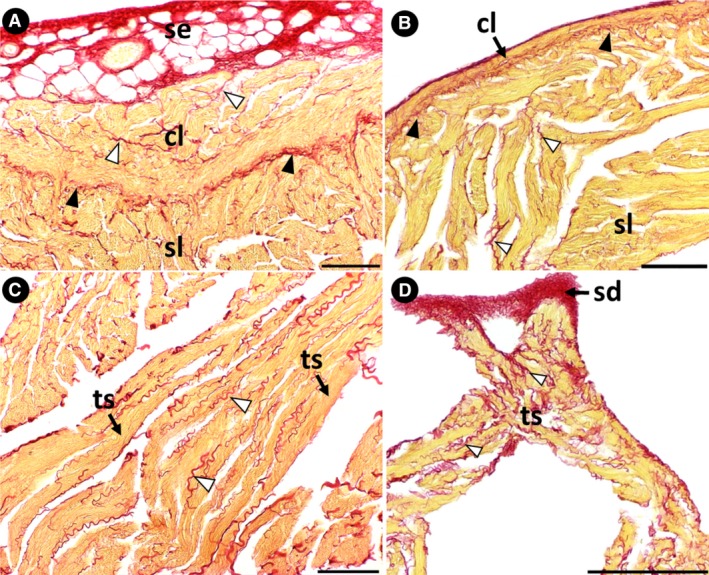
Collagen distribution in the ventricle. (A and B) Groups 3 and 2, respectively. A collagen‐rich subepicardial space surrounds the ventricle. The collagen‐rich connective tissue rim is present in the boundary between the compact and spongy layers (black arrowheads). Note the coiled collagen fibers involving the muscle bundles of the compact layer and the trabecular sheets of the spongy layer (white arrowheads). (C) Group 3. Coiled collagen fibers involve the trabecular sheets of the spongy layer (white arrowheads). (D) Group 2. A collagen‐rich subendocardial space surrounds the ventricular lumen. cl, compact layer; sd, subendocardium; se, subepicardium; sl, spongy layer; ts, trabecular sheet. Stain: Sirius red. Scale bars: (A) 100 μm; (B) 100 μm; (C) 100 μm; (D) 200 μm.
The conus arteriosus contains a compact vascularized myocardium rich in collagen fibers (Fig. 7D). This segment, surrounded by subepicardial tissue, connects the ventricle and the bulbus arteriosus (Fig. 7C,E). Anchored in the conus arteriosus by connective‐tissue extensions (Fig. 7F), the conus valves have a thick proximal base and a thinner distal portion that extends inside the bulbus arteriosus. These portions are composed of loose connective tissue with densely grouped fibroblasts surrounded by an extracellular matrix rich in collagen fibers (Fig. 7D–F). In addition, the valves have a ventricular inner layer of fibrous connective tissue facing the lumen and an outer parietal layer of fibrous connective tissue facing the conus (Fig. 7D). The compact myocardium observed in both the atrioventricular segment and the conus arteriosus occurred in fish belonging to all three groups, regardless of the presence of compact myocardium in the ventricle.
Figure 7.
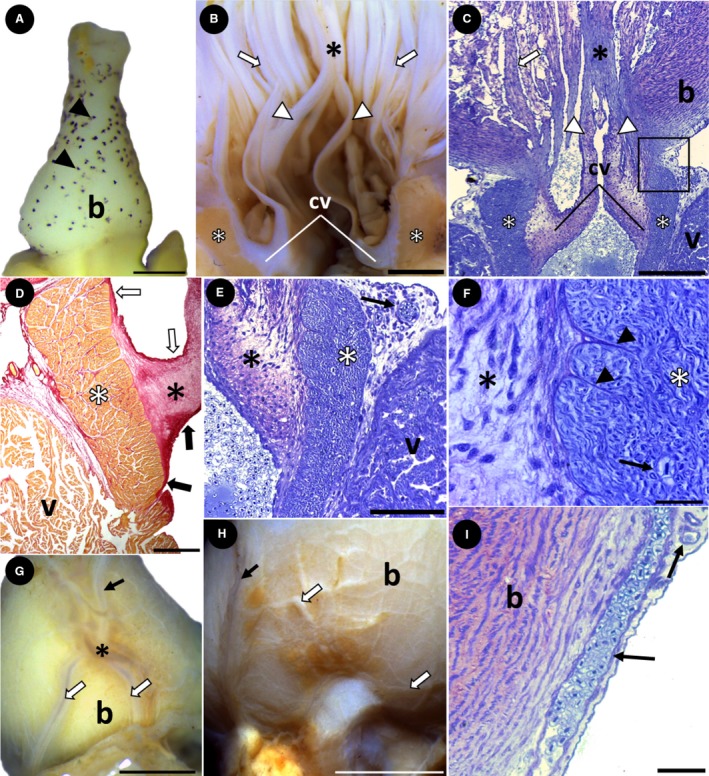
Bulbus arteriosus structure and superficial coronary arteries. (A) Group 1. Pyriform bulbus arteriosus with surface pigmentation (black arrowheads) (lateral view). (B and C) Groups 3 and 1, respectively. Anatomy and histology of the interior of the bulbus arteriosus, showing several longitudinal ridges (white arrows). The bases of the conus valves are attached to the conus arteriosus (white asterisks) and the distal ends (white arrowheads) are attached to a prominent ridge (black asterisk). The square indicates the presence of myocardium, connective tissue and the outer epicardium in the transition between the ventricle and bulbus. (D) Histological section showing the conus valves (black asterisk) anchored to the conus arteriosus (white asterisk). The fibrous connective tissue involves the outer (white arrows) and inner (black arrows) parts of the conus valves, which are continuous with the conus arteriosus. (E,F) Group 1. Histological section showing the structure of the conus arteriosus (white asterisk) and conus valves (black asterisk). The conus is composed of compact myocardium, whereas the valves are composed of loose connective tissue that attaches in the conus (black arrowheads). Note the vascular profile in the subepicardial space (E) and inside the conus (F) (black arrows). (G) Group 2. Bulbus arteriosus, showing a main coronary artery (black arrow) bifurcating on its surface (asterisk), originating the right and left coronary arteries (white arrows) (ventral view). (H) Group 3. The main coronary artery (black arrow) also originates several small branches on the surface of the bulbus arteriosus (white arrows) (lateral view). (I) Group 1. Histological section showing coronary vessels arranged longitudinally and transversely, running into the bulbus arteriosus subepicardium (black arrows). b, bulbus arteriosus; cv, conus valves; v, ventricle. Stains: (C,E,F,I) Toluidine blue; (D) Sirius red. Scale bars: (A) 0.5 mm; (B) 2 mm; (C) 200 μm; (D) 500 μm; (E) 100 μm; (F) 20 μm; (G) 2 mm; (H) 5 mm; (I) 30 μm.
The pyriform bulbus arteriosus was observed in specimens of group 1, with abundant pigmentation over its entire surface (Figs 1A and 7A). During A. gigas development, the bulbus became rounder, with no pigmentation, as evidenced in groups 2 and 3 (Fig. 1B,C). Internally, a general characteristic is the presence of several longitudinal ridges, particularly two prominent ridges, dorsal and ventral, that connect the two distal portions of the conus valves (Fig. 7B,C).
In the coronary vascularization, a main coronary artery is initially observed on the ventral surface of the bulbus arteriosus (Fig. 7G). After a short course, this artery bifurcates into two main branches that descend toward the base of the ventricle (Fig. 7G,H). The main coronary artery also originates several small branches that spread over the entire surface of the bulbus arteriosus (Fig. 7H,I). Once the main branches reach the ventricle, they spread over its entire surface. At this point, in specimens of group 1, small superficial coronary vessels penetrate only the subepicardial region. In specimens of groups 2 and 3, the coronary vessels also penetrate the myocardium, but only to the level of the compact layer (Fig. 5G). Even in semithin sections, no coronary vessels were identified in the spongy layer.
Discussion
In the caudal portion of the heart, the atrium of A. gigas showed slight external changes in shape among the groups, was dorsal to the ventricle and contacted the outflow tract laterally. The proximity of the atrium to the outflow tract is related to the distance between the inflow and outflow orifices of the ventricle, which appears to be a frequent feature in other air‐breathing species (Santer et al. 1983; Icardo, 2017). However, we did not detect any connective tissue between the walls of these components (Icardo, 2017).
The ventricle undergoes external anatomical changes, from saccular with rounded edges in low‐weight specimens of group 1 to pyramidal with angular borders and a well‐defined apex in more‐developed specimens of groups 2 and 3. Simultaneously with the external anatomical changes, a compact layer forms in the periphery of the myocardium, composed of muscle bundles in two orientations. According to the literature, the compaction of the ventricle in fish is determined mainly by the heart workload and also has a genetic basis (Farrell et al. 2012). The compact layer increases in thickness as the fish grows, as observed in the ventricle of rainbow trout (Kitoh & Oguri, 1984; Farrell et al. 1988). In A. gigas, the compact layer also increases as the fish grow, appearing in specimens of group 2 and increasing in thickness in group 3, similar to the sequence in strictly water‐breathing fish.
From a functional point of view, the pyramidal shape increases the capacity of the ventricle to generate systolic pressure, because the apexes have a shorter curvature radius (Farrell & Jones, 1992). This type of ventricle is usually associated with the presence of a compact layer, which also provides support for the ventricular systole. Generally, this layer is thicker and structurally more complex in fast‐swimming species such as members of the families Salmonidae and Scombridae (Basile et al. 1976; Santer & Greer Walker, 1980; Farrell & Jones, 1992). In this context, Tang et al. (2018) studied the heart development and morphology in two different populations of Astyanax mexicanus, one troglomorph (cave‐dwelling) and the other epigean (surface). Both populations had a pyramidal ventricle with a compact layer, independently of the swimming habit. However, the more active surface population showed a more pointed pyramidal ventricle with a higher ratio of compact myocardium compared with the less active cave‐dwelling fish. Tang et al. (2018) noted that the morphological differences coincide with functional differences in the heart between the populations, with the troglomorphs having a slower heart rate than the epigean fish.
Our study found that A. gigas possesses a ventricular morphology similar to that found in active species. However, due to its preference for lentic environments and the absence of reproductive migration, this species is classified as sedentary (Isaac et al. 1993; Castello, 2008). An association between the pyramidal shape and a compact layer in sedentary species was previously observed in Hoplias malabaricus (Gardinal et al. 2018) and in the cave‐dwelling population of Astyanax mexicanus (Tang et al. 2018) but does not appear to be a common association for the teleost heart. Therefore, although A. gigas does not have an active lifestyle, the pyramidal ventricle associated with the compact layer in the myocardium could be necessary to support its rapid growth rate (Castello, 2008).
An effective way to classify the fish ventricle was proposed by Tota et al. (1983), and revised by Durán et al. (2015) and Farrell & Smith (2017). In this classification, the authors used the relationship between the myoarchitecture and the distribution of coronary vessels to group the ventricle into four main types. However, as demonstrated for Hydrolagus affinis, some species may have a morphology that does not fit any of the types described (Durán et al. 2015). Of the four types, type II ventricles are characterized by the presence of a mixed myocardium associated with coronary vessels that are restricted to the compact layer. Based on our results, we can classify the ventricle of A. gigas as type II. Only the type II ventricle has been observed in Neotropical freshwater teleosts, even in sedentary species (Simões et al. 2002; Gardinal et al. 2018; Tang et al. 2018). However, since little published information is available, more studies are necessary to understand the evolution of the ventricle and the coronary circulation in species from this region, which harbors the highest taxonomic and functional diversity of freshwater fish (Toussaint et al. 2016).
In teleosts, the trabeculated myocardium (spongy layer) is formed by a complex arrangement of trabecular sheets delimiting lacunae that radiate from the main central lumen, originating smaller lacunae toward the periphery of the ventricle (Munshi et al. 2001; Icardo et al. 2005; Icardo, 2012, 2017). According to the authors, the numerous orifices of the trabecular sheets aid in blood ejection during systole and in contacting the individual trabeculae. However, in A. gigas, the spongy layer is organized differently. In all specimens, the trabecular sheets were organized to delimit lacunae with a similar shape and distribution in both the center and periphery, lending the spongy layer a net‐like appearance. Taking into account the function of the spongy layer in increasing the surface area of the cardiac muscle that contacts the blood (Davie & Farrell, 1991), the net‐like organization in A. gigas could provide a more uniform blood distribution through the full extent of this layer, increasing the efficiency of oxygen use by the trabeculated myocardium. Another role of the spongy layer in teleosts is to dissipate ventricular stress. The main lumen would support the highest stress, with the stress being progressively attenuated toward the periphery of the ventricle as the lacunae become smaller (Icardo et al. 2005). However, it is not known how the net‐like arrangement would function.
Regarding the heart metabolism of A. gigas, Hochachka et al. (1978) found abundant adipose tissue in the subepicardium, in addition to intracellular lipid droplets located near the mitochondria of muscle fibers, which were specialized for oxidative metabolism. According to these authors, although myocardial cells contain glycogen, lipid would be used as the main metabolic fuel (reviewed by Graham, 1997). Enzyme studies showed that the myocardium can oscillate between glycogen fermentation in limiting oxygen levels and fat catabolism when oxygen supplies are abundant. Therefore, as A. gigas starts to benefit from air‐breathing early in development, which increases the venous blood oxygen content, a trabecular arrangement that allows a more uniform blood distribution in the myocardium could directly benefit the fat‐catabolism process.
In addition to the characteristics of the ventricle, the bulbus arteriosus had numerous longitudinal ridges extending along its entire length, with two prominent main ridges, ventral and dorsal, connecting the distal portions of the conus valves. Lorenzale et al. (2017) described the same arrangement in O. bicirrhosum and suggested that the elasticity of the ridges, combined with the presence of smooth muscle, allows the ridges to play an active role in opening and closing the valves. Since this arrangement has been found so far only in the bulbus arteriosus of A. gigas and O. bicirrhosum, both belonging to the monophyletic group of Osteoglossomorpha, the question arises as to whether this feature could be a synapomorphy of this group.
Another characteristic common to both species was the existence of pigments on the surface of the bulbus arteriosus. The pigment‐containing cells in O. bicihossum are present only in the subepicardium of juvenile specimens (Lorenzale et al. 2017). For A. gigas, we observed the pigments in low‐weight specimens (group 1), but they disappeared completely in more developed individuals (groups 2 and 3). Thus, as both species are from the same order and share some common features of the bulbus structure, the disappearance of the pigments in A. gigas may also occur in O. bicihossum during its development. For other species, the pigmentation may be completely absent or distributed over the entire surface of the heart (Reyes‐Moya et al. 2015). However, in all cases, the physiological role of this pigment remains unknown (Reyes‐Moya et al. 2015; Lorenzale et al. 2017).
In conclusion, the heart of A. gigas contains a ventricle that undergoes external anatomical changes from saccular to pyramidal, associated with the progressive development of a compact layer in the periphery of the myocardium as the fish grows. The coronary vascularization is very evident, penetrating the ventricular wall to the level of the compact layer. These features allow classification of the ventricle as type II, as observed in other Neotropical freshwater teleosts. The trabeculated myocardium has a net‐like organization that differs from other teleosts described in the literature. The presence of this arrangement in all the groups studied here indicates that it could play an important role in blood distribution in the trabeculated myocardium, benefiting the fat‐catabolism process previously described in the literature.
Conflict of interest
All authors are aware of the policy and have no conflict of interest to declare.
Author contributions
Mario Vitor Buzete Gardinal and Thalles Fernando Rocha Ruiz performed the morphological procedures, interpreted the data and drafted the manuscript. Sandro Estevan Moron, Eliane Tie Oba Yoshioka and Ligia Uribe Gonçalves provided the fish and aided in the morphological procedures and interpreting data. Irene Bastos Franceschini Vicentini and Carlos Alberto Vicentini aided in interpreting data and writing the final draft. All authors read and approved the final version of this article.
Acknowledgements
The authors thank the Laboratory of Morphology of Aquatic Organisms, Faculty of Science, Bauru Campus and the Aquaculture Center of UNESP – CAUNESP for technical assistance. They also thank the Electron Microscopy Laboratory of the Faculty of Agrarian and Veterinary Sciences of UNESP, Jaboticabal Campus for contributions in ultrastructural procedures. We are grateful to the referees for their helpful comments. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001 (Process No. 157704).
References
- Bancroft JD, Layton C (2013) Connective and mesenchymal tissues with their stains. In: Bancroft's Theory and Practice of Histological Techniques (eds. Suvarna SK, Layton C, Bancroft JD.), pp. 187–214. London: Churchill Livingstone. [Google Scholar]
- Basile C, Goldspink G, Modigh M, et al. (1976) Morphological and biochemical characterization of the inner and outer ventricular myocardial layers of adult tuna fish (Thunnus thynnus L). Comp Biochem Physiol B 54, 279–283. [DOI] [PubMed] [Google Scholar]
- Castello L (2008) Lateral migration of Arapaima gigas in floodplains of the Amazon. Ecol Freshw Fish 17, 38–46. [Google Scholar]
- Davie PS, Farrell AP (1991) The coronary and luminal circulations of the myocardium of fishes. Can J Zool 69, 1993–2001. [Google Scholar]
- Durán AC, Lopez‐Unzu MA, Rodríguez C, et al. (2015) Structure and vascularization of the ventricular myocardium in Holocephali: their evolutionary significance. J Anat 226, 501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell AP, Jones DR (1992) The heart In: Fish Physiology, Vol 12A: The Cardiovascular System (eds Hoar WS, Randall DJ, Farrell AP.), pp. 1–88. New York: Academic Press. [Google Scholar]
- Farrell AP, Smith F (2017) Cardiac form, function and physiology In: Fish Physiology, Vol 36A: The Cardiovascular System: Morphology, Control and Function (eds. Gamperl K, Gillis TE, Farrell AP, et al.), pp. 155–264. Cambridge, MA: Academic Press. [Google Scholar]
- Farrell AP, Hammons AM, Graham M (1988) Cardiac growth in rainbow trout, Salmo gairdneri . Can J Zool 66, 2368–2373. [Google Scholar]
- Farrell AP, Farrell ND, Jourdan H, et al. (2012) A perspective on the evolution of the coronary circulation in fishes and the transition to terrestrial life In: Ontogeny and Phylogeny of the Vertebrate Heart (eds. Sedmera D, Wang T.), pp. 75–102. New York: Springer. [Google Scholar]
- Gardinal MVB, Faccioli CK, Chedid RA, et al. (2018) Myocardium arrangement and coronary vessels distribution in the ventricle of three Neotropical freshwater teleosts. Zool Sci 35, 360–366. [DOI] [PubMed] [Google Scholar]
- Graham JB. (ed.) (1997) Air‐Breathing Fishes: Evolution, Diversity, and Adaptation. San Diego: Academic Press. [Google Scholar]
- Hochachka PW, Randall DJ (1978) Alpha helix Amazon expedition, September–October 1976. Can J Zool 56, 713–716. [Google Scholar]
- Hochachka PW, Guppy M, Guderley H, et al. (1978) Metabolic biochemistry of water‐ vs air‐breathing osteoglossids: heart enzymes and ultrastructure. Can J Zool 56, 759–768. [Google Scholar]
- Icardo JM (2012) The Teleost heart: a morphological approach In: Ontogeny and Phylogeny of the Vertebrate Heart (eds. Sedmera D, Wang T.), pp. 35–53. New York: Springer. [Google Scholar]
- Icardo JM (2017) Heart morphology and anatomy In: Fish Physiology, Vol 36A: The Cardiovascular System: Morphology, Control and Function (eds. Gamperl K, Gillis TE, Farrell AP, Brauner CJ.), pp. 1–54. Cambridge, MA: Academic Press. [Google Scholar]
- Icardo JM, Imbrogno S, Gattuso A, et al. (2005) The heart of Sparus auratus: a reappraisal of cardiac functional morphology in teleosts. J Exp Zool Part A Comp Exp Biol 303, 665–675. [DOI] [PubMed] [Google Scholar]
- Isaac VJ, Rocha VL, Mota S (1993) Considerações sobre a legislação da ‘piracema’ e outras restrições da pesca da região do Médio Amazonas In: Povos das Águas, Realidade e Perspectivas na Amazônia. (eds Furtado LG, Leitão W, Fiuza Mello A.), pp. 188–211. Belém: Ministério de Ciência e Tecnologia, Conselho Nacional de Pesquisa, Museu Paraense Emilio Goeldi. [Google Scholar]
- Junqueira LCU, Bignolas G, Brentani RR (1979) Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11, 447–455. [DOI] [PubMed] [Google Scholar]
- Kitoh K, Oguri M (1984) Differentiation of the compact layer in the heart ventricle of rainbow trout. Bull Japan Soc Sci Fish 51, 539–542. [Google Scholar]
- Lorenzale M, López‐Unzu MA, Fernández MC, et al. (2017) Anatomical, histochemical and immunohistochemical characterization of the cardiac outflow tract of the silver arowana, Osteoglossum bicirrhosum (Teleostei: Osteoglossiformes). Zoology 120, 15–23. [DOI] [PubMed] [Google Scholar]
- Munshi JSD, Olson KR, Roy PK, et al. (2001) Scanning electron microscopy of the heart of the climbing perch. J Fish Biol 59, 1170–1180. [Google Scholar]
- Reyes‐Moya I, Torres‐Prioris A, Sans‐Coma V, et al. (2015) Heart pigmentation in the gray bichir, Polypterus senegalus (Actinopterygii: Polypteriformes). Anat Hist Embryol 44, 475–480. [DOI] [PubMed] [Google Scholar]
- Sánchez‐Quintana D, García‐Martínez V, Climent V, et al. (1996) Myocardial fiber and connective tissue architecture in the fish heart ventricle. J Exp Zool A Ecol Genet Physiol 275, 112–124. [Google Scholar]
- Santer RM (1985) Morphology and innervation of the fish heart. Adv Anat Embryol Cell Biol 89, 89–102. [DOI] [PubMed] [Google Scholar]
- Santer RM, Greer Walker M (1980) Morphological studies on the ventricle of teleost and elasmobranch hearts. J Zool 190, 259–272. [Google Scholar]
- Santer RM, Greer Walker M, Emerson L, et al. (1983) On the morphology of the heart ventricle in marine teleost fish (Teleostei). Comp Biochem Physiol A Physiol 76, 453–457. [Google Scholar]
- Satchell GH (1970) A functional appraisal of the fish heart. Fed Proc 29, 1020–1023. [PubMed] [Google Scholar]
- Simões K, Vicentini CA, Orsi AM, et al. (2002) Myoarchitecture and vasculature of the heart ventricle in some freshwater teleosts. J Anat 200, 467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang JLY, Guo Y, Stockdale WT, et al. (2018) The developmental origin of heart size and shape differences in Astyanax mexicanus populations. Dev Biol 441, 272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tota B (1989) Myoarchitecture and vascularization of the elasmobranch heart ventricle. J Exp Zool 252, 122–135. [Google Scholar]
- Tota B, Cimini V, Salvatore G, et al. (1983) Comparative study of the arterial and lacunary systems of the ventricular myocardium of elasmobranch and teleost fishes. Am J Anat 167, 15–32. [DOI] [PubMed] [Google Scholar]
- Tota B, Cerra MC, Mazza R, et al. (1997) The heart of the Antarctic icefish as paradigm of cold adaptation. J Therm Biol 22, 409–417. [Google Scholar]
- Toussaint A, Charpin N, Brosse S et al. (2016) Global functional diversity of freshwater fish is concentrated in the Neotropics while functional vulnerability is widespread. Sci Rep, 6, 22125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Val AL, Almeida‐Val VMF (1995) The Amazon ichthyofauna In: Fishes of the Amazon and Their Environment (eds. Val AL, Almeida‐Val VMF.), pp. 28–69. Berlin: Springer. [Google Scholar]
- Val AL, Silva MNP, Almeida‐Val VMF (1998) Hypoxia adaptation in fish of the Amazon: a never‐ending task. Afr Zool 33, 107–114. [Google Scholar]


