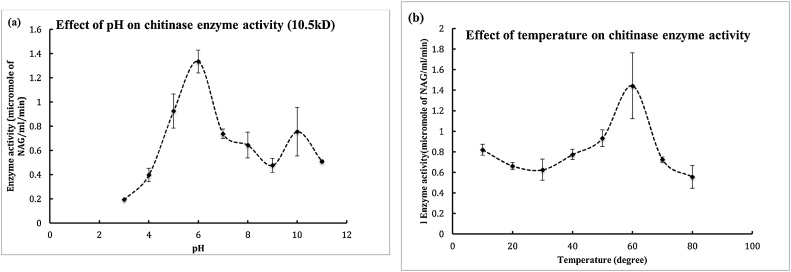
Fig. 2.
(a) The partially purified enzyme was checked for its activity at different incubation temperature with the substrate. Briefly, the active fractions of DEAE Sephacel and DEAE Sepharose were pooled together. 100 μl of this enzyme was mixed with 100 μl of appropriate pH buffer i.e 50 mM sodium acetate buffer, 50 mM citrate buffer, 50 mM Tris HCl buffer, 50 mM glycine NaOH buffer(followed by 200 μl of substrate (1% clloidal chitin).The reaction mixture was then incubated at 37 for 2 h. The rest process was carried out according to standard assay conditions i.e after incubation the reaction mixture was centrifuged, the supernatant was collected and 100 μl of potassium tetra borate was added to it followed by boiling for 3 min. To this DMAB reagent was added at RT the colour development was measured at 585 nm. (b) The partially purified enzyme was checked for its activity at different incubation temperature with the substrate. Briefly, the active fractions of DEAE Sephacel and DEAE Sepharose were pooled together. 100 μl of this enzyme was mixed with 100 μl of water followed by 200 μl of substrate (1% colloidal chitin).The reaction mixture was then incubated at 37 for 2 h. The rest process was carried out according to standard assay conditions i.e after incubation the reaction mixture was centrifuged, the supernatant was collected and 100 μl of potassium tetraborate was added to it followed by boiling for 3 min. To this DMAB reagent was added at RT the colour development was measured at 585 nm.