Figure 5.
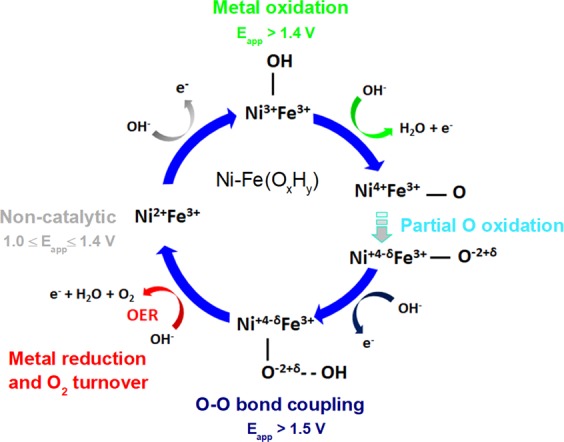
Mechanistic scheme summarizing the metal oxidation observed in our in situ XAS measurements of the Ni65Fe35(OxHy) catalyst. The resting state (non-catalytic potential) is observed between 1.0–1.4 V vs. RHE. Above this potential (>1.4 V) the oxidation of Ni2+ to Ni3+/4+ occurs. An oxidation state lower than +4 is usually observed both in Ni(OOH) and in mixed Ni-Fe(OxHy) catalysts. In the 1st metal oxidation step (shown in green), a discharge of an OH− group takes place, which adsorbs to a vacant site and increases the oxidation state by one unit. In this step a radical species may instead be formed (-OH∙) which would not result in a metal oxdation state increase (this is not shown in our cycle however may happen at both Fe and Ni sites). In the 2nd metal oxidation step, Ni is further oxidized to Ni4+. However, if there is a consecuetive step where charge re-distribution occurs from O 2p → Ni 3d, the average metal oxidation state is lowered (Ni+4-δ), and as a consequence the oxygen oxidation state is increased (O−2+δ). This step according to our O K-edge data is a stable state and is strongly correlated to the metal oxidation step, and occurs prior to O-O bond formation.
