Abstract
Background: Atrial fibrillation (AF) is the most common cardiac arrhythmia whose incidence is on the rise globally. However, the pathophysiologic mechanism of AF remains poorly understood and there has been a lack of circulatory markers to diagnose and predict prognosis of AF. In the present study, by measuring metabolic profile and analyzing plasma amino acid levels in AF patients, we sought to determine whether amino acid metabolism was correlated to the occurrence of AF. Methods: Consecutive patients admitted to hospital for AF were enrolled. Plasma samples were obtained after overnight fast and a profile of 61 amino acids was then measured using gas chromatography/mass spectrometry (GC/MS). Results: Twenty-three AF and thirty-seven control patients were enrolled in the study. A number of plasma amino acids were altered in AF, which showed significant prediction value for AF. Intriguingly, circulating 4-hydroxypyrrolidine-2-carboxylic was gradually lowered with the persistence of AF. Plasma amino acid levels were more strongly correlated with each other in AF as compared with control. Conclusion: By utilizing non-target metabolic profile surveys, we have found a number of altered amino acids, which exhibit diagnostic value for AF. Enhanced amino acids correlation network further identified AF as a metabolism disorder.
Keywords: Atrial Fibrillation, Metabolism Network, Plasma Amino Acid Profile
Introduction
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia and its incidence is increasing globally [1]. Patients with AF have increased risks of death, heart failure, hospitalization, and thromboembolic events [2,3]. However, the pathophysiological mechanism underlying AF remains underexplored, and there has been a lack of circulatory markers for diagnosing AF and predicting its prognosis [4].
The pathogenesis of AF is well known to be related to alterations in the anatomy and electrophysiology of the heart. Common risk factors for AF include a genetic predisposition, heart structural alterations, and myocardial ischemia [5]. It is worth noting that while metabolic diseases including those related to alcohol intake, diabetes mellitus, and hyperthyroidism are also associated with an increased incidence of AF [6–8], it has not been established whether alteration of the metabolism profile is related to the pathogenesis of AF.
Patients with hyperthyroidism have an increased risk of developing AF [9,10]. The biologically active thyroid hormones include thyroxin (T4) and 3,5,3′-triiodothyronine (T3), which are composed of a phenyl ring attached via an ether linkage to a tyrosine molecule [11,12]. The biosynthesis and metabolism of thyroid hormones involve several amino acids [13]. Previous studies have found that excess thyroid hormone increased the likelihood of AF in experimental animals, even in the presence of a beta receptor and vagal blockade [14]. However, it is unclear whether circulating amino acid levels are directly related to the incidence of AF in patients with normal thyroid function [12]. This situation means that exploring the amino acid profile in AF patients has important clinical implications, since this may affect whether or not AF is also regarded as a metabolic disorder, and whether certain specific plasma amino acids could be used to diagnose AF and predict its prognosis.
Metabolomics has emerged as a powerful tool for defining changes in both global and cardiac-specific metabolism that occur across a spectrum of cardiovascular disease states [15]. The advanced metabolites measurement technology allows for simultaneous quantitation of a wide variety of molecular intermediates from multiple bioenergetic pathways. Previous studies have shown several metabolic biomarkers to be associated with prevalent and incident AF [16–18], but it is controversial whether the metabolomics alteration could be further validated [19]. In addition, few studies have investigated the metabolic difference in paroxysmal and persistent AF.
In the present study, we measured the metabolic profile and analyzed plasma amino acid levels in AF patients with the aim of determining if amino acid metabolism is correlated with the occurrence of AF.
Methods
Study design and population
Consecutive patients admitted to the cardiology department of the First Affiliated Hospital of Xi’an Jiaotong University for AF between March 2016 and June 2016 were selected. AFs were diagnosed upon initial admission. The diagnosis of AF was made according to 2014 ACC/AHA guidelines [20]. A patient could only be included once. Written informed consent was obtained from all study participants, with ethnic committee approval at the First Affiliated Hospital of Xi’an Jiaotong University. The present study complied with the Declaration of Helsinki, and formal consent was obtained from all patients.
Inclusion and exclusion criteria
The inclusion criteria were: (1) age between 18 and 80 years old; (2) clinical diagnosis of AF; and (3) informed consent for the present study. The exclusion criteria were: (1) hypothyroid or hyperthyroid disease; (2) active hepatitis or end stage liver failure; (3) severe non-cardiac disease with expected survival of less than 1 year; (4) other health behaviors that might affect the study, i.e., dementia, intemperance, etc; and (5) unwillingness to participate.
Demographic and biochemical measurements
Subjects who participated in the physical examination were interviewed by trained interviewers to complete a questionnaire that included questions, such as age, sex, history of disease(s), drug use, regular exercise, and alcohol consumption. Further information about present medication and a detailed medical history were obtained via hospital medical records. All participants donated blood samples after ≥10-h overnight fasting for biochemical measurement, including complete blood count, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides (TG), fasting blood glucose (FBG), serum creatinine (CRE), blood urea nitrogen (BUN), serum uric acid (UA), cyscatin-c, homocysteine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), alkaline phosphatase (ALP), thyroid function, and anticoagulation function. Additionally, after an overnight fast, venous blood was withdrawn the next morning after admission and immediately centrifuged at 3000 rpm for 10 min at 4°C. Plasma was separated and stored at −80°C until further processing.
Determination of plasma amino acids
Plasma samples were obtained from 23 AF patients and 37 respective controls. After an overnight fast, venous blood was withdrawn the next morning after admission and immediately centrifuged at 3000 rpm for 10 min at 4°C. Plasma was separated and stored at −80°C until further processing. Before analysis, plasma samples were collected, thawed, and 20 μl added to a microcentrifuge tube containing 10 μl of 0.1 mM L-Norvaline and 70 μl of acetonitrile. Samples were vortexed for 20 s and centrifuged for 10 min at a speed of 15000 g/min. Amino acid profile was then measured using gas chromatography/mass spectrometry (GC/MS) [21]. A total of 61 serum amino acid metabolites was measured (Supplementary Table S1).
Statistics
Data were normalized using MetaboAnalyst before analyses [22–31] (Supplementary Figure S1). Amino acids level between AF and control was compared using Student’s t test. Amino acids level among paroxysmal and persistent AF and control was compared using one-way ANOVA. Receiver operating characteristics (ROC) was used and areas under the ROC curve were calculated to compare the effectiveness of different amino acids to identify AF. Pearson’s analysis was performed to compare correlation between amino acids and biochemical indicators using SPSS 17.0. Pearson’s analysis performed to compare correlation between each amino acid and heatmap was created using R studio. P-values <0.05 were considered as significant *<0.05, **<0.01, and ***<0.001.
Results
Baseline characteristics
A total of 60 patients were enrolled in the study, 23 AF and 37 control patients. Table 1 describes the demographic and biochemical characteristics of the AF and control patients. The mean age was 62.15 ± 14.18 years in AF and 61.62 ± 7.99 years in control patients. No significant difference in risk factors at baseline were seen between AF and control except for heart rate, creatine, and pro-brain natriuretic peptide (proBNP).
Table 1. Baseline characteristics of the patients in the cohort.
| AF (n=23) | Control (n=37) | P-value | |
|---|---|---|---|
| Age (y) | 62.15 ± 14.18 | 61.62 ± 7.99 | ns |
| Female | 8 | 11 | ns |
| Hear rate (bpm) | 80.76 ± 15.06 | 72.21 ± 10.76 | <0.05 |
| sBP (mmHg) | 120.00 ± 14.32 | 133.15 ± 19.79 | ns |
| dBP (mmHg) | 75.65 ± 10.25 | 75.47 ± 11.49 | ns |
| EF (%) | 60.25 ± 11.87 | 63.74 ± 9.33 | ns |
| AST (U/l) | 23.24 ± 5.52 | 30.40 ± 47.19 | ns |
| ALT (U/l) | 25.69 ± 13.00 | 25.54 ± 15.60 | ns |
| CRE (mg/dl) | 8.01 ± 2.62 | 7.27 ± 1.22 | <0.05 |
| UA (mmol/l) | 342.58 ± 90.87 | 282.97 ± 84.11 | ns |
| TC (mg/dl) | 142.33 ± 35.27 | 153.66 ± 37.57 | ns |
| TG (mg/dl) | 135.19 ± 105.14 | 161.47 ± 162.15 | ns |
| HDL-C (mg/dl) | 40.03 ± 9.77 | 42.45 ± 14.69 | ns |
| LDL-C (mmol/l) | 1.99 ± 0.80 | 2.22 ± 0.75 | ns |
| proBNP (ng/ml) | 2616.93 ± 7274.73 | 251.58 ± 553.39 | <0.05 |
| FT4 (mmol/l) | 16.73 ± 3.43 | 16.26 ± 2.68 | ns |
| FT3 (mmol/l) | 5.12 ± 1.04 | 5.03 ± 0.88 | ns |
| TSH (mmol/l) | 2.50 ± 2.28 | 1.92 ± 1.17 | ns |
| Current/ex smoker (%) | 23.53% | 44.12% | |
| Current/ex drinker (%) | 11.76% | 29.41% | |
| DM (%) | 17.39% | 29.73% | |
| Hypertension (%) | 47.06% | 67.65% | |
| CHF (%) | 5.88% | 0.00% | |
| MI (%) | 11.76% | 11.76% |
Abbreviations: BP, blood pressure; CHF, chronic heart failure; TC, total cholesterol; DM, diabetes mellitus; EF, ejection fraction; FT3, free triiodothyronine; FT4, free thyroxine; MI, myocardial infarction; TSH, thyroid stimulating hormone.
Plasma amino acid profile of AF patients
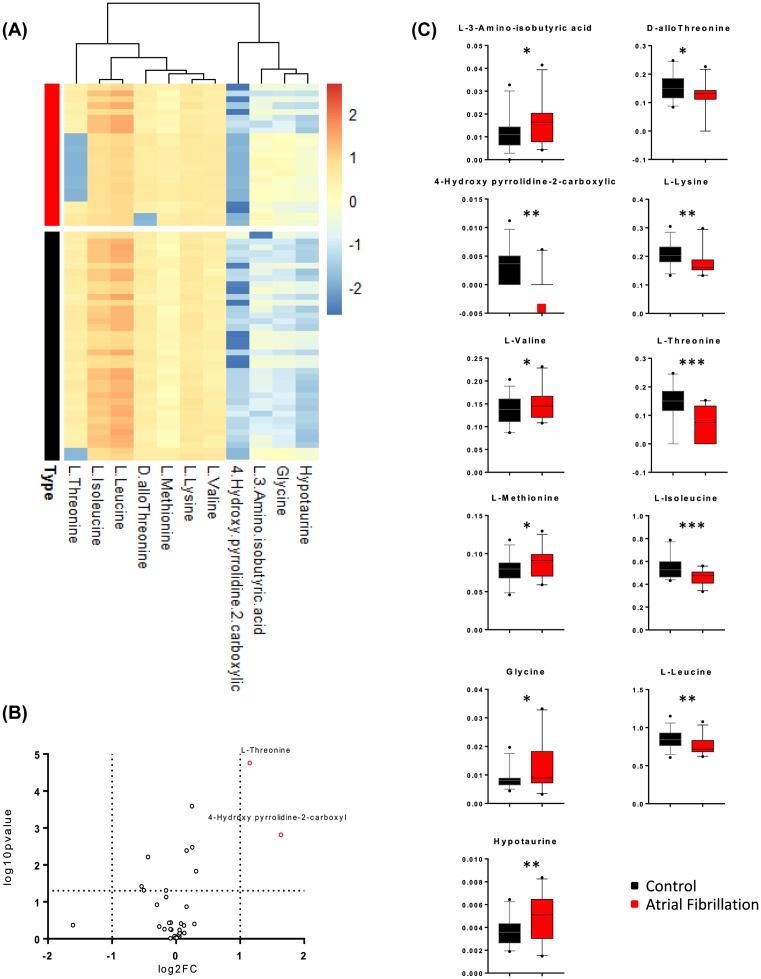
Amino acid levels were compared between AF and control patients using Student’s t test, which identified significant differences in the levels of L-3-aminoisobutyric acid, D-allothreonine, 4-hydroxy-pyrrolidine-2-carboxylic acid (4HP2C), L-lysine, L-valine, L-threonine, L-methionine, L-isoleucine, glycine, L-leucine, and hypotaurine. A heatmap of the individual levels of significantly altered plasma amino acids (P<0.05) is shown in Figure 1A, and Figure 1B shows the overall level of each significantly altered amino acid. A general overview of the plasma amino acid levels is provided in Figure 1C based on those amino acids with relative changes exceeding 2 and P-values under 0.05. Among the above-listed amino acids that were significantly altered, only 4HP2C and L-threonine showed changes larger than 2-fold.
Figure 1. Expression profile of amino acids in patients with AF as compared with control.
(A) Heatmap of significantly altered plasma amino acids (P<0.05) in AF and control patients. The colors in the heatmap indicated the log-2-transformed values of each amino acids. (B) The general overview of plasma amino acids levels, when we select amino acid with fold change beyond 2 and P-value under 0.05. The y-axis represented log-10-transformed P-value for each amino acid, and the x-axis represented log-2-transformed fold change for each amino acid. Red dots stood for significantly altered amino acids with more than two times of the fold change. (C) Illustration of the significantly altered amino acids in control and AF group. Data were analyzed using the Student’s ttest. Mean ± s.e.m. *P< 0.05, **P< 0.01, and ***P<0.001.
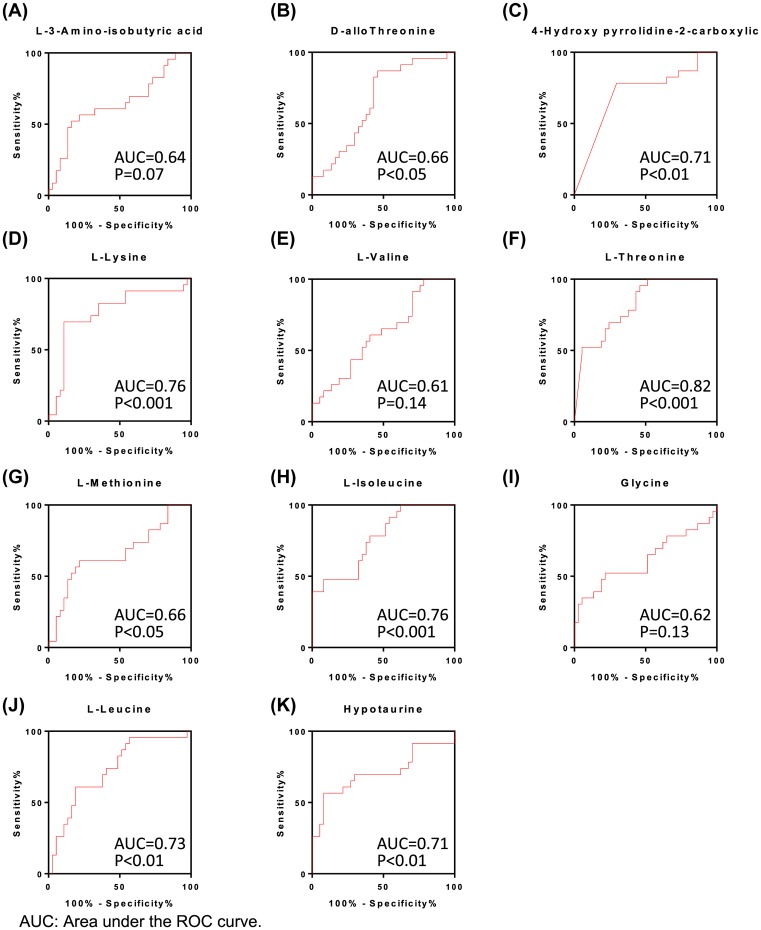
ROC analysis of different amino acids for identifying AF
The alterations in circulating amino acids indicated that amino acid levels could be a potential diagnostic marker for AF. To further identify the diagnostic value of the above-listed amino acids, ROC analysis was performed for each individual amino acid. The area under the ROC curve exceeded 0.6 for all 11 of the significantly altered amino acids, while the P-value was significant for D-allothreonine, 4HP2C, L-lysine, L-threonine, L-methionine, L-isoleucine, L-leucine, and hypotaurine (Figure 2). The ROC analysis therefore further validated the prognostic value of the plasma levels of amino acids in AF. We also performed an automated identification and performance evaluation of important features using three multivariate algorithms: support-vector machines, partial-least-squares discriminant analysis, and random forests using the MetaboAnalyst software. This additional analysis further validated the diagnostic value of the above-listed amino acids (Supplementary Figure S3).
Figure 2. ROC analysis of different amino acids to identify AF.
(A–K) ROC curve analysis for respective amino acids. Area under the ROC curve and P-value for each amino acid were shown in each figure.
Relative levels of amino acid in controls, AF, paroxysmal AF, and persistent AF
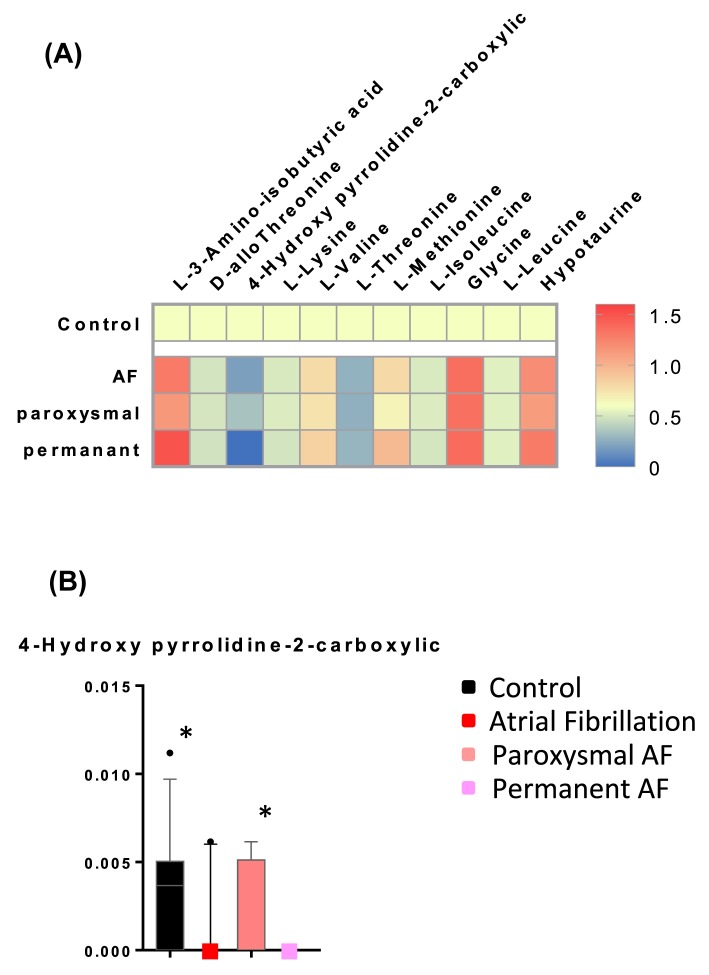
Since previous AF guidelines have divided AF into paroxysmal and persistent AF [32], we further compared the plasma amino acid levels between paroxysmal and persistent AF patient groups. The 23 AF patients comprised 13 with paroxysmal and 10 with persistent AF. Figure 3A shows the levels of significantly altered amino acids in total AF, paroxysmal AF, and persistent AF relative to controls. Only the circulating level of 4HP2C showed a significant alteration in paroxysmal AF relative to persistent AF. However, it is worth noting that 4HP2C was not detectable in any of the ten patients with persistent AF (Figure 3B), suggesting that 4HP2C gradually decreases with the progression of AF.
Figure 3. Relative level of amino acids in control, AF, paroxysmal AF, and persistent AF.
(A) Heatmap of relative average amino acid in AF, paroxysmal AF, and persistent AF as compared with control patients. The colors in the heatmap indicated the relative amino acid levels in each group as compared with control. (B) 4-Hydroxypyrrolidine-2-carboxylic levels in control, total AF, paroxysmal AF, and persistent AF patients. Data were analyzed using one-way ANOVA. Mean ± s.e.m. *P< 0.05.
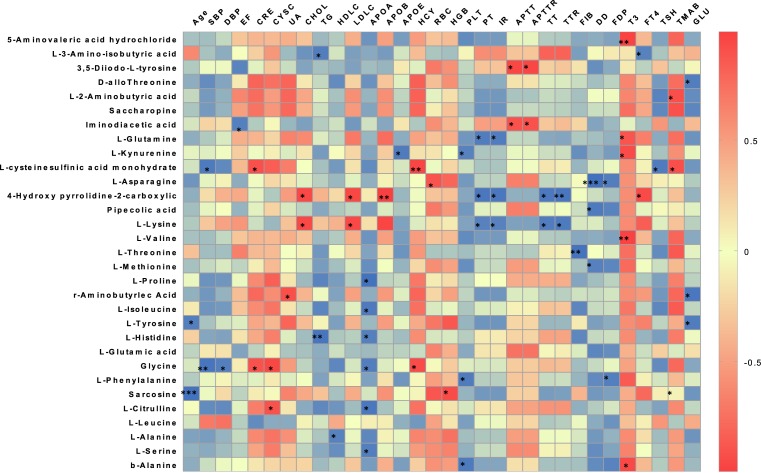
Association between amino acid and clinical characteristics
To further assess the relationships between alterations of the amino acid profile and the pathogenesis of AF, the correlations between age, blood pressure, heart function, lipid levels, renal function, coagulation function, and thyroid function were analyzed (Figure 4). 4HP2C and L-lysine were found to be significantly and positively correlated with TC and LDL-C levels, while 4HP2C and L-lysine, together with L-glutamine, L-kynurenine, L-asparagine, pipecolic acid, L-threonine, L-methionine, and L-phenylalanine were found to be significantly negatively correlated with coagulation function.
Figure 4. Association of amino acids level and clinical factors.
Correlations between age, blood pressure, heart function, lipid levels, renal function, coagulation function, and amino acid profile in AF patients. The colors in the heatmap stood for efficiency of the Pearson’s correlation. *P<0.05, **P<0.01, and ***P<0.001.
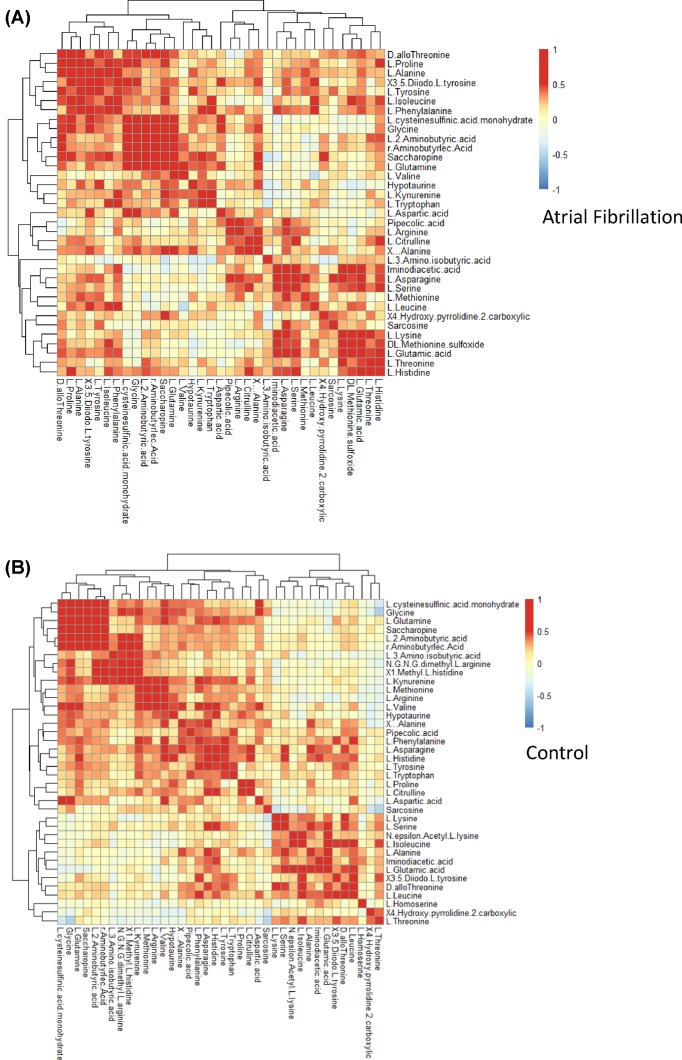
Analysis of correlation of plasma amino acid levels between AF and controls
In order to identify interactions of plasma amino acids, we performed a correlation analysis in AF and control patients. Figure 5A,B shows heatmaps of the Pearson’s correlation coefficients between the respective amino acids, with red blots indicating the highest positive coefficient of 1 and blue blots indicating the lowest negative coefficient of −1. The amino acid levels exhibited stronger positive correlations with AF patients than with controls. We then selected the amino acid pairs with correlation coefficients higher than 0.7 or lower than −0.7 to identify strongly correlated amino acids. L-2-aminobutyric acid, saccharopine, L-glutamine, L-cysteine sulfinic acid monohydrate, R-aminobutyric acid, and glycine showed stronger correlations with AF than with controls (Supplementary Figure S4A,B). The respective correlation networks for AF and controls are shown in Supplementary Figure S4C,D.
Figure 5. Correlation analysis of plasma amino acids between AF and control.
(A) Correlations between each amino acid in AF patients. (B) Correlations between each amino acid in control patients. The colors within each crossover represented the correlation efficiency between the respective amino acids. Blue color indicated decreased correlation and red color indicated enhanced correlation.
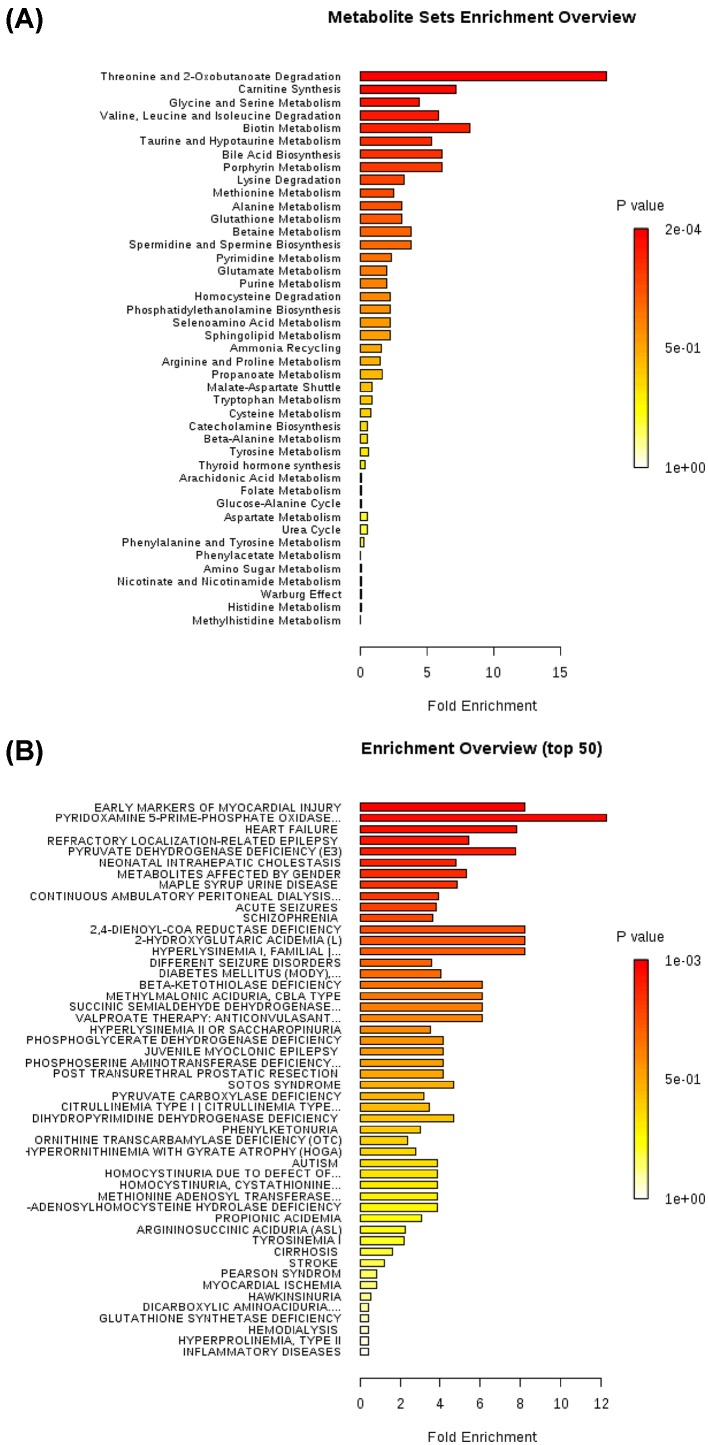
Enrichment analysis of plasma amino acids based on pathway and disease
Finally, we performed an enrichment analysis using MetaboAnalyst to identify sets of amino acids that could be grouped based on their involvement in the same biological pathways (Figure 6A and Supplementary Table S2) or disease phenotypes (Figure 6B and Supplementary Table S3). The strongest association was found between AF and the threonine and 2-oxobutanoate degradation pathway (P<0.001, FDR q<0.001). Also, early markers of myocardial injuries were found to be significantly increased in AF (P<0.001, FDR q<0.001).
Figure 6. Enrichment analysis of plasma amino acids based on pathway and disease.
(A) Enrichment analysis of plasma amino acids based on pathway. The strongest association was found between AF and threonine and 2-oxobutanoate degradation pathway (P-value <0.001, FDR q<0.001) in the biological pathways. (B) Enrichment analysis of plasma amino acids based on disease. The strongest association was found between AF and early markers of myocardial injuries (P-value <0.001, FDR q<0.001).
Discussion
The present study analyzed the metabolic profile and network of plasma amino acids in AF patients. Amino acid metabolism was found to be altered in AF based on the following observations: (1) several plasma amino acids were altered in AF, most of which also showed significant prediction value for AF; (2) the circulating level of 4-hydroxypyrrolidine-2-carboxylic gradually decreased in the presence of persistent AF; and (3) plasma amino acid levels were more strongly correlated with each other in AF than in controls.
AF was traditionally considered a heart rhythm disorder caused by disorganized electrical impulses usually originating in the roots of the pulmonary veins [33,34]. However, there is accumulating evidence for a correlation between metabolic disorders and the occurrence of AF [6–8]. In the present study, we used a selective analytic platform to perform an in-depth investigation of amino acid metabolism. We investigated metabolite levels in AF and control patients in order to identify biomarkers that are correlated with the incidence of AF. Our initial analysis revealed differentially regulated amino acids, which further confirmed the presence of pathogenic dysfunctional metabolism in AF. The ROC analysis further identified several circulatory markers that show prognostic value for AF. Thus, the findings of the present study suggest that plasma amino acid levels are potential biomarkers for diagnosing or predicting AF.
We have also shown that the circulating level of 4HP2C is significantly decreased in the presence of persistent AF. 4HP2C is biosynthetically derived from the amino acid proline, which is a non-essential amino acid in the body that can be synthesized from L-glutamate. Proline is reportedly involved in the synthesis of collagen and fibrosis in the human body [35]. Since the plasma L-proline level was not significantly altered in our AF patients, we speculate that a defect in proline metabolism could contribute to the pathogenesis of AF. Moreover, in addition to electrophysiological alterations, patients with AF also displayed elevated levels of atrial fibrous tissue, along with increased expression levels of collagen I and III [36]. Thus, identifying that altered amino acids could lead to dietary or other interventions that are effective therapeutic targets for AF.
The main novelty of the present study is its investigation of the pathogenesis of AF from a metabolomics perspective and the description of metabolic alteration in AF based on a non-targeted metabolomics approach. Although it is well established that age, alcohol intake, and hyperthyroidism can cause AF [6–8], the precise underlying mechanisms are still poorly understood. The present findings point to the utility of non-targeted metabolic profile surveys for identifying predictors of clinical diseases [19,21], and we have applied such a survey to 61 amino acids. The correlation matrix in Figure 4 shows significant correlations between various amino acids and clinical factors. The separate correlation matrices in Figure 5 for AF and control patients show all of the correlations of amino acids within each group. It is noteworthy that interactions between amino acids play an important role in the pathogenesis and development of AF. Identifying the basis for these interactions could be helpful for improving the diagnosis of AF and predicting its prognosis.
Recent advances in metabolic profiling technologies have enhanced the feasibility of high-throughput patient screening for diagnosing various disease states [37,38], and an increasing number of studies are utilizing metabolomics approaches to identify metabolic alterations and pathogenic factors in AF [39–42]. However, one previous study has not found altered metabolites in AF, making the metabolic regulation in AF controversial [19]. In our study, we have further validated the metabolic alteration in AF patients, focusing on significantly altered amino acids levels. Moreover, as compared with paroxysmal AF, the circulating level of 4HP2C is significantly decreased in the presence of persistent AF. The present study could potentially add to our diagnostic armamentarium for AF patients.
The main limitation of the present study is that it had a case–control observational design. Since relatively few patients were enrolled, selection and observation bias might be present. Further investigations are therefore warranted to investigate metabolic alterations in AF and between paroxysmal and persistent AF based on larger cohorts. Moreover, the plasma amino acid levels were measured using non-targeted metabolic methods, which restrict precise evaluations of amino acid levels. We therefore suggest that these findings also require further investigation. Finally, animal studies and clinical trials are required to further validate the predictive function of single amino acids and to exclude compensatory actions.
In conclusion, the present study has established that measuring the levels of plasma amino acids can improve the ability to predict AF. Altered levels of amino acids have diagnostic value for AF, and the analysis of amino acid correlations further identified AF as a metabolism disorder. Our data suggest that plasma amino acids are potential circulatory markers for diagnosing AF and that therapeutic strategies of amino acid supplementation should be considered.
Clinical perspectives
The pathophysiologic mechanism of AF remains underexplored, and there has been a lack of circulatory markers to diagnose and predict prognosis of AF.
Altered amino acids exhibit diagnostic value for AF and enhanced amino acids correlation network further identifies AF as a metabolism disorder.
Our data suggest that plasma amino acids could be potential circulatory markers for diagnosing AF and therapeutic strategies of amino acids supplementation could be considered as a potential treatment.
Supporting information
Supplementary Figure 1.
Data normalization.
Supplementary Figure 2.
Heatmap of the overall amino acid.
Supplementary Figure 3.
Multivariate Exploratory ROC Analysis.
Supplementary Figure 4.
Correlation network of amino acids between AF and Control. A. Heatmap of the strongly correlated amino acids in AF patients. B. Heatmap of the strongly correlated amino acids in control patients. The figures within each crossover represented the correlation efficiency between the respective amino acids. Purple color indicated enhanced co-expression and yellow color indicated decreased co-expression. Correlation threshold was set as 0.7. C. and D. The respective correlation network for AF and control patients.
Supplementary Table 1. List of plasma amino acids being tested.
Supplementary Table 2. Enrichment Analysis of metabolite sets associated with pathways.
Supplementary Table 3. Enrichment Analysis of metabolite sets associated with disease.
Abbreviations
- 4HP2C
4-hydroxypyrrolidine-2-carboxylic
- AF
atrial fibrillation
- ALT
alanine aminotransferase
- AST
aspartate transaminase
- CRE
creatine
- HDL-C
high density lipoprotein cholesterol
- LDL-C
low density lipoprotein cholesterol
- ROC
receiver operating characteristic
- TC
total cholesterol
- TG
triglyceride
- TSH
thyroid stimulating hormone
- UA
urea acid
Funding
The present study was supported by Central University Basic Science Foundation of China [grant number 1191329724]; National Natural Science Foundation of China [grant numbers 81570406, 81500219]; the Fundamental Research Funds of the First Affiliated Hospital of Xi’an Jiaotong University [grant number 2017QN-25]; and the Natural Science Foundation of Shaanxi province [grant number 2017JM8016].
Author contribution
J.Q.S., M.Y.G., H.B.L., J.H.L., X.L., P.N.L., B.Z., S.M.L, Y.Y.D., B.W.L., and Y.W. conducted the experiments and revised the work. Y.W., J.Q.S., C.F.S., and Z.Y.Y. designed the experiments and wrote the paper.
Competing interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.Sirish P., Li N., Timofeyev V., Zhang X.D., Wang L., Yang J. et al. (2016) Molecular mechanisms and new treatment paradigm for atrial fibrillation. Circ. Arrhythm. Electrophysiol. 9, e003721, 10.1161/CIRCEP.115.003721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel N.J., Deshmukh A., Pant S., Singh V., Patel N., Arora S. et al. (2014) Contemporary trends of hospitalization for atrial fibrillation in the United States, 2000 through 2010: implications for healthcare planning. Circulation 129, 2371–2379 10.1161/CIRCULATIONAHA.114.008201 [DOI] [PubMed] [Google Scholar]
- 3.Chugh S.S., Blackshear J.L., Shen W.K., Hammill S.C. and Gersh B.J. (2001) Epidemiology and natural history of atrial fibrillation: clinical implications. J. Am. Coll. Cardiol. 37, 371–378 10.1016/S0735-1097(00)01107-4 [DOI] [PubMed] [Google Scholar]
- 4.Camm A.J., Kirchhof P., Lip G.Y., Schotten U., Savelieva I. and et al. (2010) Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur. Heart J. 31, 2369–2429 10.1093/eurheartj/ehq278 [DOI] [PubMed] [Google Scholar]
- 5.Debonnaire P., Joyce E., Hiemstra Y., Mertens B.J., Atsma D.E. et al. (2017) Left atrial size and function in hypertrophic cardiomyopathy patients and risk of new-onset atrial fibrillation. Circ. Arrhythm. Electrophysiol. 10, e004052, 10.1161/CIRCEP.116.004052 [DOI] [PubMed] [Google Scholar]
- 6.Gore-Panter S.R., Rennison J.H. and Van Wagoner D.R. (2017) Genetic-genomic insights into the metabolic determinants of spontaneous atrial fibrillation. Circ. Arrhythm. Electrophysiol. 10, e005636, 10.1161/CIRCEP.117.005636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quintanilla J.G., Perez-Villacastin J., Perez-Castellano N., Pandit S.V., Berenfeld O., Jalife J. et al. (2016) Mechanistic approaches to detect, target, and ablate the drivers of atrial fibrillation. Circ. Arrhythm. Electrophysiol. 9, e002481 10.1161/CIRCEP.115.002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong C.X., Sun M.T., Odutayo A., Emdin C.A., Mahajan R., Lau D.H. et al. (2016) Associations of epicardial, abdominal, and overall adiposity with atrial fibrillation. Circ. Arrhythm. Electrophysiol. 9, e004378, 10.1161/CIRCEP.116.004378 [DOI] [PubMed] [Google Scholar]
- 9.Frost L., Vestergaard P. and Mosekilde L. (2004) Hyperthyroidism and risk of atrial fibrillation or flutter: a population-based study. Arch. Intern. Med. 164, 1675–1678 10.1001/archinte.164.15.1675 [DOI] [PubMed] [Google Scholar]
- 10.Krahn A.D., Klein G.J., Kerr C.R., Boone J., Sheldon R. et al. (1996) How useful is thyroid function testing in patients with recent-onset atrial fibrillation? The Canadian Registry of Atrial Fibrillation Investigators Arch. Intern. Med. 156, 2221–2224 10.1001/archinte.1996.00440180083010 [DOI] [PubMed] [Google Scholar]
- 11.Hennemann G., Docter R., Friesema E.C., de Jong M., Krenning E.P. and Visser T.J. (2001) Plasma membrane transport of thyroid hormones and its role in thyroid hormone metabolism and bioavailability. Endocr. Rev. 22, 451–476 10.1210/edrv.22.4.0435 [DOI] [PubMed] [Google Scholar]
- 12.Chen Y.C., Chen S.A., Chen Y.J., Chang M.S., Chan P. and Lin C.I. (2002) Effects of thyroid hormone on the arrhythmogenic activity of pulmonary vein cardiomyocytes. J. Am. Coll. Cardiol. 39, 366–372 10.1016/S0735-1097(01)01731-4 [DOI] [PubMed] [Google Scholar]
- 13.Spitzweg C., Heufelder A.E. and Morris J.C. (2000) Thyroid iodine transport. Thyroid 10, 321–330 10.1089/thy.2000.10.321 [DOI] [PubMed] [Google Scholar]
- 14.Arnsdorf M.F. and Childers R.W. (1970) Atrial electrophysiology in experimental hyperthyroidism in rabbits. Circ. Res. 26, 575–581 10.1161/01.RES.26.5.575 [DOI] [PubMed] [Google Scholar]
- 15.Shah S.H., Kraus W.E. and Newgard C.B. (2012) Metabolomic profiling for the identification of novel biomarkers and mechanisms related to common cardiovascular diseases: form and function. Circulation 126, 1110–1120 10.1161/CIRCULATIONAHA.111.060368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayr M., Yusuf S., Weir G., Chung Y.L., Mayr U., Yin X. et al. (2008) Combined metabolomic and proteomic analysis of human atrial fibrillation. J. Am. Coll. Cardiol. 51, 585–594 10.1016/j.jacc.2007.09.055 [DOI] [PubMed] [Google Scholar]
- 17.De Souza A.I., Cardin S., Wait R., Chung Y.L., Vijayakumar M., Maguy A. et al. (2010) Proteomic and metabolomic analysis of atrial profibrillatory remodelling in congestive heart failure. J. Mol. Cell. Cardiol. 49, 851–863 10.1016/j.yjmcc.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 18.Alonso A., Yu B., Qureshi W.T., Grams M.E., Selvin E., Soliman E.Z. et al. (2015) Metabolomics and incidence of atrial fibrillation in African Americans: the Atherosclerosis Risk in Communities (ARIC) study. PLoS ONE 10, e0142610 10.1371/journal.pone.0142610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko D., Riles E.M., Marcos E.G., Magnani J.W., Lubitz S.A., Lin H. et al. (2016) Metabolomic profiling in relation to new-onset atrial fibrillation (from the Framingham Heart study). Am. J. Cardiol. 118, 1493–1496 10.1016/j.amjcard.2016.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.January C.T., Wann L.S., Alpert J.S., Calkins H., Cigarroa J.E., Cleveland J.C. Jr et al. (2014) 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 130, 2071–2104 10.1161/CIR.0000000000000040 [DOI] [PubMed] [Google Scholar]
- 21.Song Y., Xu C., Kuroki H., Liao Y. and Tsunoda M. (2018) Recent trends in analytical methods for the determination of amino acids in biological samples. J. Pharm. Biomed. Anal. 147, 35–49 10.1016/j.jpba.2017.08.050 [DOI] [PubMed] [Google Scholar]
- 22.Xia J. and Wishart D.S. (2016) Using MetaboAnalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinformatics 55, 14.10.1–14.10.91, 10.1002/cpbi.11 [DOI] [PubMed] [Google Scholar]
- 23.Xia J., Sinelnikov I.V., Han B. and Wishart D.S. (2015) MetaboAnalyst 3.0–making metabolomics more meaningful. Nucleic Acids Res. 43, W251–W257 10.1093/nar/gkv380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia J., Mandal R., Sinelnikov I.V., Broadhurst D. and Wishart D.S. (2012) MetaboAnalyst 2.0–a comprehensive server for metabolomic data analysis. Nucleic Acids Res. 40, W127–W133 10.1093/nar/gks374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xia J. and Wishart D.S. (2011) Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protoc. 6, 743–760 10.1038/nprot.2011.319 [DOI] [PubMed] [Google Scholar]
- 26.Xia J. and Wishart D.S. (2011) Metabolomic data processing, analysis, and interpretation using MetaboAnalyst. Curr. Protoc. Bioinformatics 34, 14.10.1–14.10.48 10.1002/0471250953.bi1410s34 [DOI] [PubMed] [Google Scholar]
- 27.Xia J., Psychogios N., Young N. and Wishart D.S. (2009) MetaboAnalyst: a web server for metabolomic data analysis and interpretation. Nucleic Acids Res. 37, W652–W660 10.1093/nar/gkp356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia J., Broadhurst D.I., Wilson M. and Wishart D.S. (2013) Translational biomarker discovery in clinical metabolomics: an introductory tutorial. Metabolomics 9, 280–299 10.1007/s11306-012-0482-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia J., Sinelnikov I.V. and Wishart D.S. (2011) MetATT: a web-based metabolomics tool for analyzing time-series and two-factor datasets. Bioinformatics 27, 2455–2456 10.1093/bioinformatics/btr392 [DOI] [PubMed] [Google Scholar]
- 30.Xia J. and Wishart D.S. (2010) MetPA: a web-based metabolomics tool for pathway analysis and visualization. Bioinformatics 26, 2342–2344 10.1093/bioinformatics/btq418 [DOI] [PubMed] [Google Scholar]
- 31.Xia J. and Wishart D.S. (2010) MSEA: a web-based tool to identify biologically meaningful patterns in quantitative metabolomic data. Nucleic Acids Res. 38, W71–W77 10.1093/nar/gkq329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allessie M.A., Boyden P.A., Camm A.J., Kleber A.G., Lab M.J., Legato M.J. et al. (2001) Pathophysiology and prevention of atrial fibrillation. Circulation 103, 769–777 10.1161/01.CIR.103.5.769 [DOI] [PubMed] [Google Scholar]
- 33.Thijssen V.L., Ausma J., Liu G.S., Allessie M.A., van Eys G.J. and Borgers M. (2000) Structural changes of atrial myocardium during chronic atrial fibrillation. Cardiovasc. Pathol. 9, 17–28 10.1016/S1054-8807(99)00038-1 [DOI] [PubMed] [Google Scholar]
- 34.Wijffels M.C., Kirchhof C.J., Dorland R., Power J. and Allessie M.A. (1997) Electrical remodeling due to atrial fibrillation in chronically instrumented conscious goats: roles of neurohumoral changes, ischemia, atrial stretch, and high rate of electrical activation. Circulation 96, 3710–3720 10.1161/01.CIR.96.10.3710 [DOI] [PubMed] [Google Scholar]
- 35.Albaugh V.L., Mukherjee K. and Barbul A. (2017) Proline precursors and collagen synthesis: biochemical challenges of nutrient supplementation and wound healing. J. Nutr., 147, 2011–2017 10.3945/jn.117.256404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Polyakova V., Miyagawa S., Szalay Z., Risteli J. and Kostin S. (2008) Atrial extracellular matrix remodelling in patients with atrial fibrillation. J. Cell. Mol. Med. 12, 189–208 10.1111/j.1582-4934.2008.00219.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis G.D., Wei R., Liu E., Yang E., Shi X., Martinovic M. et al. (2008) Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J. Clin. Invest. 118, 3503–3512 10.1172/JCI35111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson L.C., Martens C.R., Santos-Parker J.R., Bassett C.J., Strahler T.R. et al. (2018) Amino acid and lipid associated plasma metabolomic patterns are related to healthspan indicators with aging in humans. Clin. Sci., 132, 1765–1777 10.1042/CS20180409 [DOI] [PubMed] [Google Scholar]
- 39.Rusnak J., Behnes M., Saleh A., Fastner C., Sattler K., Barth C. et al. (2018) Interventional left atrial appendage closure may affect metabolism of essential amino acids and bioenergetic efficacy. Int. J. Cardiol., 268, 125–131 10.1016/j.ijcard.2018.05.031 [DOI] [PubMed] [Google Scholar]
- 40.Jung Y., Cho Y., Kim N., Oh I.Y., Kang S.W., Choi E.K. et al. (2018) Lipidomic profiling reveals free fatty acid alterations in plasma from patients with atrial fibrillation. PLoS ONE 13, e0196709 10.1371/journal.pone.0196709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fastner C., Behnes M., Sartorius B., Wenke A., Lang S., Yucel G. et al. (2018) Interventional left atrial appendage closure affects the metabolism of acylcarnitines. Int. J. Mol. Sci. 19, e500, 10.3390/ijms19020500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sattler K., Behnes M., Barth C., Wenke A., Sartorius B., El-Battrawy I. et al. (2017) Occlusion of left atrial appendage affects metabolomic profile: focus on glycolysis, tricarboxylic acid and urea metabolism. Metabolomics 13, 127 10.1007/s11306-017-1255-2 [DOI] [PMC free article] [PubMed] [Google Scholar]