Abstract
Our previous study demonstrated that bromodomain-containing protein 7 (BRD7) inhibits cell proliferation and tumor growth, restoring the expression of B-cell lymphoma 2 antagonist/killer (Bak) sensitized breast cancer cells to paclitaxel. However, the association between BRD7 and paclitaxel sensitization, as well as BRD7 and Bak in breast cancer remains unknown. In the present study, immunochemical staining was performed to measure the expression of BRD7 and Bak in breast cancer tissues. Cell Counting Kit-8 assay, flow cytometry and tumor xenograft procedures were performed to evaluate the biological role of BRD7 and Bak in breast cancer cells. Western blotting, reverse transcription-quantitative polymerase chain reaction, chromatin immunoprecipitation and luciferase reporter assays were also performed. BRD7 was positively correlated with Bak levels in breast cancer tissues, and the survival rate of patients with low Bak and BRD7 expression was significantly lower than that of patients with high Bak and BRD7 expression. In addition, BRD7 activated Bak promoter activity and induced Bak expression in an indirect manner. Furthermore, ectopic expression of BRD7 inhibited cell proliferation, tumor growth and sensitized cancer cells to paclitaxel, while knockdown of Bak abolished BRD7-mediated inhibitory effects on cell proliferation and paclitaxel sensitization in breast cancer cells whether in vitro and in vivo. The results demonstrated that BRD7 inhibits cell proliferation and sensitizes breast cancer cells to paclitaxel by activating Bak; they also provide promising targets for the diagnosis and treatment of breast cancer.
Keywords: B-cell lymphoma 2-antagonist/killer protein, bromodomain-containing protein 7, chemoresistance, breast cancer
Introduction
B-cell lymphoma 2 (Bcl2)-antagonist/killer protein (Bak) is a member of the BH3-only Bcl-2 protein family. Bak is a proapoptotic effector of canonical mitochondrial apoptosis (1). BH3-only Bcl-2 proteins promote apoptosis by directly activating Bak, and by suppressing antiapoptotic proteins in the mitochondria and endoplasmic reticulum (ER). These proteins are regulated by several mechanisms, including transcription and post-translational modifications, in order to prevent constitutive cell death (2). Bak exerts its proapoptotic activity via hierarchical and highly regulated interactions with other BH3-only Bcl-2 family members; in addition, it also operates as a sensor of extrinsic and intrinsic cell death signals (3). Our previous study demonstrated that high Bak expression was associated with favorable prognosis in breast cancer; furthermore, the restoration of Bak sensitized breast cancer cells to paclitaxel (4). Therefore, molecules that mimic the BH3 domain are proposed to be molecular targets for cancer therapy. Therefore, investigations to obtain further knowledge regarding the molecular mechanisms that regulate responsiveness to cancer therapy using these molecules are warranted (5).
Bromodomain-containing protein 7 (BRD7), a member of the bromodomain-containing protein family, has been identified as a tumor suppressor in several types of cancer, including nasopharyngeal carcinoma, breast and prostate cancers (6). BRD7 inhibits cancer cell growth and metastasis, and promotes apoptosis by downregulating the phosphatase and tensin homolog/protein kinase B (AKT), mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (ERK) and retinoblastoma/E2F transcription factor signaling pathways (7–11). Furthermore, it is involved in multiple physiological processes, including the regulation of spermatogenesis and inflammation (12,13). BRD7 acts as a co-activator for p53 and is involved in the acetylation of p53 target genes and in the subsequent activation of functional p53 pathways (14,15). The depletion of BRD7 in breast cells results in the loss of estrogen receptor α (ERα) expression, and this loss of ERα is reflected in resistance to the antiestrogen drug Fulvestrant (16). Bak functions as a downstream effector of p53 in the mitochondria (17). The accumulation of phospho-p53 in the mitochondria activates Bak, which subsequently induces cell apoptosis and death (18,19). However, the association between BRD7 and Bak remains unknown.
In the present study, the association and functions of BRD7 and Bak in breast cancer tissues were investigated, and the regulatory effects of BRD7 on Bak were also determined. BRD7 and Bak were downregulated in breast cancer tissues, and there was a positive correlation between their expression. BRD7 activated Bak promoter activity and induced Bak expression in an indirect manner. In addition, ectopic expression of BRD7 in breast cancer cells inhibited cell proliferation, promoted apoptosis and sensitized cancer cells to paclitaxel, while restoring the expression of Bak abolished BRD7-mediated inhibitory effects on cell proliferation and paclitaxel sensitization in breast cancer cells, whether in vitro and in vivo. These results demonstrated that BRD7 inhibits cell proliferation and sensitizes breast cancer cells to paclitaxel by activating Bak; thus, these results may provide a target for the diagnosis and treatment of breast cancer.
Materials and methods
Tissue samples and clinical data
A total of 225 breast cancer tissues (age, 46±0.66 years; male/female patient ratio, 1/224) and 62 non-tumor breast tissues (age, 48±0.84 years; n=62 female patients) were collected from the Second Xiangya Hospital of Central South University (Hunan, China) between November 2001 and September 2012. Patients with diabetes, nephritis, hepatitis or cardiovascular disease were excluded. Patient information was obtained from medical records. The present study was approved by the Ethics Committee of Central South University. Written informed consent was obtained from all of the participants.
Cell culture
The human breast cancer cell lines MCF-7 and MDA-MB-231 were obtained from American Type Culture Collection (Manassas, VA, USA). All cells were cultured in high glucose Dulbecco's modified Eagle's medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and maintained in a humidified atmosphere of 5% CO2 in air at 37°C.
Cell treatment
To achieve BRD7 overexpression in MCF-7 and MDA-MB-231 cells, cells at 80% confluence were transfected in 6-well plates with pIRES2-EGFP-3Flag/BRD7 or pIRES2-EGFP (Addgene, Inc., Cambridge, MA, USA) using Lipofectamine 3000™ transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The cells transfected with pIRES2-EGFP were used as a negative control. Then, to knockdown Bak expression in MCF-7 and MDA-MB-231 cells, the cells were transfected with Bak small interfering RNA (siRNA) oligonucleotides or control siRNA oligonucleotides (siRNA sequence, 5′-CCGACGCUAUGACUCAGAGdTdT-3′; Guangzhou RiboBio Co., Ltd., Guangzhou, China; 50 nmol/l) using Lipofectamine 3000™ transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. The cells transfected with control siRNA oligonucleotides were used as a negative control. To determine the chemosensitivity in MCF-7 and MDA-MB-231 cells 48 h following BRD7 overexpression or Bak knockdown, the cells were treated with paclitaxel (Corden Pharma Latina S.P.A, Sermoneta, Latina, Italy; 400 nM for MCF-7 and 80 nM for MDA-MB-231) for 48 h at 37°C.
Immunohistochemistry and evaluation of staining
The tumor sections were fixed with 4% paraformaldehyde at room temperature for 48 h, paraffin-embedded and then serially cut at 4 µm. Slides were regularly deparaffinized with xylene twice (each for 10 min), rehydrated through an ethanol gradient (100, 95, 90, 80 and 70%, each for 5 min), and washed with phosphate-buffered saline (PBS) twice. Slides were retrieved in citric acid buffer (pH 6.0) by boiling for 20 min using a microwave oven. Following cooling, the slices were washed with PBS and then blocked with 100% normal goat serum (Boster Biological Technology, Pleasanton, CA, USA), and incubated with the following primary antibodies overnight at 4°C: Rabbit polyclonal anti-BRD7 (1:100 dilution; cat. no. 51009-2-AP; ProteinTech Group, Inc., Chicago, IL, USA) and rabbit polyclonal anti-Bak (1:200 dilution; cat. no. 3814; Cell Signaling Technology, Inc., Danvers, MA, USA). The sections were then washed with PBS and incubated with an horseradish peroxidase (HRP)-conjugated anti-rabbit secondary antibody (1:1 dilution; cat. no. KIT-5905; Fuzhou Maixin Biotech Co., Ltd., Fujian, China) for 2 h at 37°C. Then the sections were washed with PBS and stained using a 3,3′-diaminobenziden detection kit (Maxim Biomedical, Inc., Rockville, MD, USA). Finally, the sections were counterstained with hematoxylin for 30 sec at room temperature. The evaluation of staining was performed as previously described (4). Briefly, the slides were evaluated by two independent pathologists blinded to clinicopathological features and the clinical course, under a light microscope (BX51; Olympus Corporation, Tokyo, Japan). The staining intensity of BRD7 or Bak were scored as 0 (negative, -), 1 (weak, +), 2 (moderate, ++) and 3 (strong, +++). The extent of staining was scored as 0–1.0 (0–100%). The final staining score (0–3) was calculated as the multiplication of the intensity score and extent score. A final score of ≥1 was defined as high expression, otherwise scores were defined as low expression.
Cell viability assay
MCF-7 and MDA-MB-231 cells transfected with BRD7 or Bak were plated in 96-well plates at a density of 1,000 cells in 100 µl of the aforementioned DMEM+FBS media/well. The cells were treated with paclitaxel (Corden Pharma Latina S.P.A; 400 nM for MCF-7 and 80 nM for MDA-MB-231) for 48 h at 37°C with 5% CO2, and the cell viability was assessed by Cell Counting Kit-8 assay (cat. no. B34304; Bimake, Shanghai, China) according to the manufacturers' instructions.
Flow cytometry for apoptosis analysis
An Annexin V-phycoerythrin (PE)/7-aminoactinmycin D (AAD) Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ, USA) was used to detect cell apoptosis. Following BRD7 overexpression and Bak siRNA treatment, 2×105 cells were collected and washed twice with cold PBS by centrifugation at 1,000 × g for 5 min at room temperature, and then resuspended in 100 µl of 1X binding buffer. A volume of 5 µl Annexin V-PE and 5 µl 7-AAD were added to the cell suspension, mixed well and incubated for 10 min at room temperature, protected from light. Cells were analyzed using the BD LSRFortessa flow cytometer (BD Biosciences) with FlowJo software (version 10.5; FlowJo LLC, Ashland, OR, USA). Three independent experiments were performed for this assay.
Western blotting
Cells were lysed in cold Radioimmunoprecipitation Assay buffer and protein concentrations were determined using a BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). A total of 60 µg protein was separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA), which were blocked by soaking in 5% non-fat milk for 1 h at 37°C. Then the membranes were incubated with the corresponding primary antibodies overnight at 4°C. The following antibodies were used: anti-Flag (1:2,000 dilution; cat. no. F7425; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), anti-GAPDH (1:2,000 dilution; cat. no. 60004-1-Ig; ProteinTech Group, Inc.), anti-BRD7 (1:1,000 dilution; cat. no. 51009-2-AP; ProteinTech Group, Inc.), anti-Bak (1:1,000 dilution; cat. no. 3814; Cell Signaling Technology, Inc.), anti-total poly (adenosine diphosphate-ribose) polymerase (PARP; 1:1,000 dilution; cat. no. 9532; Cell Signaling Technology, Inc.) and anti-cleaved PARP (1:1,000 dilution; cat. no. 9548; Cell Signaling Technology, Inc.). Following washing with 1X TBST (containing 0.05% Tween-20) 3 times for 8 min each, the membranes were incubated with the appropriate secondary antibody [1:3,000 dilution; cat. no. SA00001-1 for HRP-conjugated AffiniPure Goat Anti-Mouse IgG (H+L); 1:3,000 dilution; cat. no. SA00001-2 for HRP-conjugated AffiniPure Goat Anti-rabbit IgG (H+L); ProteinTech Group, Inc.] for 1 h at 37°C. Finally, the bands were visualized using an Enhanced Chemiluminescence kit (EMD Millipore). Signals were quantified by ImageJ software (version 1.8.0; National Institutes of Health, Bethesda, MD, USA) and normalized to GAPDH.
Luciferase reporter assay
To detect the promoter activity of Bak, the putative promoter region (2,000 bp upstream of the Bak transcription initiation site) was synthesized and inserted into a pGL3-enhancer vector (Promega Corporation, Madison, WI, USA) to generate a recombinant luciferase reporter gene (pGL3-Bak). MCF-7 and MDA-MB-231 cells were transfected with pGL3-Bak and co-transfected with a BRD7 expressing plasmid or vector control (Addgene, Inc.) using Lipofectamine 3000™ transfection reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions. At 48 h post-transfection, cells were collected and lysed with radioimmunoprecipitation (RIPA; cat. no. B100020, ProteinTech Group, Inc.). A volume of 100 µl of the supernatants were used to detect luciferase activities (normalized to Renilla luciferase activity) using a luciferase reporter gene assay kit (Promega Corporation) and the PARADIGM Detection Platform (Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed using a SuperScript™ IV First-Strand Synthesis System (cat. no. 18091050; Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocols to generate total cDNA to serve as templates for Bak mRNA detection. To obtain cDNA, 10 µl 2X RT Reaction Mix, 2 µl RT Enzyme Mix, 100 ng RNA and DEPC-treated water up to 20 µl were added into a tube, gently mixed and then RT-PCR was conducted with the following temperature protocol: 25°C for 10 min, 50°C for 30 min, 85°C for 5 min and then chilled on ice. The mRNA expression of Bak was measured using Bright Green 2X qPCR Master Mix (Applied Biological Materials, Inc., Richmond, Canada) according to manufacturer's instructions. The expression of β-actin was used as an endogenous control. RT-qPCR was performed with the following thermocycling conditions: 95°C for 3 min, and then 39 cycles of 95°C for 10 sec and 60°C for 30 sec. Data were quantified using the 2−ΔΔCq method (20). The primers used were as follows: Bak forward, 5′-GCAGGCTGATCCCGTCC-3′ and reverse, 5′-CAAACAGGCTGGTGGCAATC-3′; β-actin forward, 5′-TTGTTACAGGAAGTCCCTTGCC-3′ and reverse, 5′-ATGCTATCACCTCCCCTGTGTG-3′.
Chromatin immunoprecipitation (ChIP) assay
The EZ-Magna ChIP kit (EMD Millipore) was used for the ChIP assays according to manufacturer's protocol. MCF-7 cells at 100% confluency in 6-well plates were fixed with 4% paraformaldehyde for 24 h at room temperature and incubated with glycine for 10 min at room temperature to generate DNA-protein crosslinks. Then, the cells were lysed with the cell lysis buffer RIPA (cat. no. B100020; ProteinTech Group, Inc.) and sonicated to generate chromatin fragments. Next, the lysates were immunoprecipitated with Magnetic Protein A Beads conjugated with Flag-specific antibodies (1:100 dilution; cat. no. F7425; Sigma-Aldrich; Merck KGaA). The samples immunoprecipitated with immunoglobulin G were used as a negative control, and baculoviral IAP repeat containing 2 was used as a positive control, based our previous results (21). Finally, the precipitated DNA was analyzed by PCR under the following thermocycling conditions: 95°C for 3 min, followed by 29 cycles of 95°C for 10 sec and 60°C for 30 sec to amplify different regions of the Bak promoter segments. The promoter sequence was divided into 10 fragments based on the DNA walking method (22). All of these PCR products were ~200–240 bp in length. The primers of each of the promoter segment pairs were as follows: F1 forward, 5′-CCCAGCAGGGTGAGCGCC-3′ and reverse, 5′-CAGCAGTGGGGAAGGCACA-3′ (239 bp); F2 forward, 5′-TCTGTGCCTTCCCCACTGCT-3′ and reverse, 5′-GCTCTGGGAGGGGTGCAAA-3′ (239 bp); F3 forward, 5′-AGTTTGCACCCCTCCCAGA-3 and reverse, 5′-GTGGTCCAGCCCTCCTCCAC-3′ (239 bp); F4 forward, 5-GGTGGAGGAGGGCTGGACC-3′ and reverse, 5′-CATGCCCAGCTAATTTTTGTAT-3′ (237 bp); F5 forward, 5′-GAAACCCCATCTCTACTAAAAATAC-3′ and reverse, 5′-TGGGAGGCAAGCAAAACTCTT-3′ (214 bp); F6 forward, 5′-GAGTTTTGCTTGCCTCCCACC-3′ and reverse 5′-TGGATGGGGGAGGCAGAGC-3′ (232 bp); F7 forward, 5′-CCTAGCTCTGCCTCCCCCA-3′ and reverse, 5′-TGGGAGATGGGAGTGGAGGTC-3′ (214 bp); F8 forward, 5′-GGCTCTGACCTCCACTCCCAT-3′ and reverse 5′-CAGATCTCAGCAGCCCCAGC-3′ (238 bp); F9 forward, 5′-CTTGAGCTTCCCCTTCCCCA-3′ and reverse, 5′-GGAAACTGGGCTCCCACTCA-3′ (209 bp); and F10 forward, 5′-AGGGGCTGAGTGGGAGCC-3′ and reverse, 5′-CACCCTACAGGCTGTCGGC-3′ (215 bp). The PCR products were resolved electrophoretically on a 1.5% agarose gel and visualized using ethidium bromide staining.
Tumor xenograft in nude mice
Animal experiments were approved by the Ethical Committee for Animal Research of Central South University. The MCF-7 cells were transfected with BRD7 plasmids with or without Bak siRNA as aforementioned for 36 h prior to injections. To assess tumor growth and paclitaxel sensitization, 100 µl of the transfected MCF-7 cells (1×106) was subcutaneously injected into nude mice (total n=24 female mice; n=6 mice/group; age, 2 months; weight, ~15 g); the mice were housed under a 12-h light/dark cycle at 20–22°C with 50–60% humidity and had free access to food and water. The mice were purchased from Shanghai Laboratory Animal Center (Shanghai, China). The researchers who injected the cells were blind to the treatment. On day 14 following the injection of cells, when the smallest group of tumors was produced, the mice were intraperitoneally injected with paclitaxel (Corden Pharma Latina S.P.A) every 2 days, with 4 injections administered in total (15 mg/kg/injection). The control mice were injected with an equal volume of saline. The tumor sizes were measured regularly and calculated using the following formula: 0.5 × L × W2, where L and W refer to the length and width of the tumor, respectively. On day 26 following cell injections, the mice were sacrificed by injecting an overdose of pentobarbital sodium. The tumors were then excised and weighed. The inhibition rate was calculated as follows: (Average tumor weight of control group - tumor weight of the experimental group/average tumor weight of control group) × 100, where the experimental group refers to control+paclitaxel, BRD7+paclitaxel or BRD7+siBak+paclitaxel. The tumor tissues were then fixed with 4% paraformaldehyde for 24 h at room temperature and paraffin-embedded for the immunohistochemistry assay as aforementioned.
Statistical analysis
In the present study, all experiments were repeated at least three times, and data are expressed as the mean ± standard error of the mean. SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA) was used to perform statistical analysis. Differences between two groups were compared by an independent-samples t-test. Differences among three or more groups were compared by one-way analysis of variance with a post hoc Bonferroni test. The correlation between BRD7 and Bak in breast cancer tissues was analyzed by Pearson's correlation analysis. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of BRD7 and Bak in breast cancer
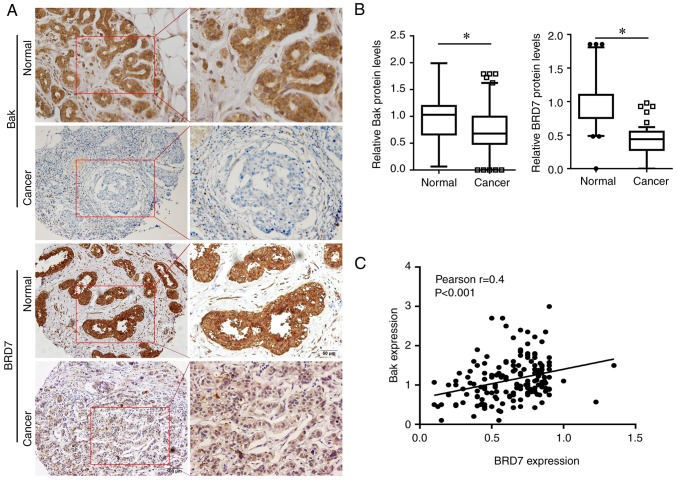
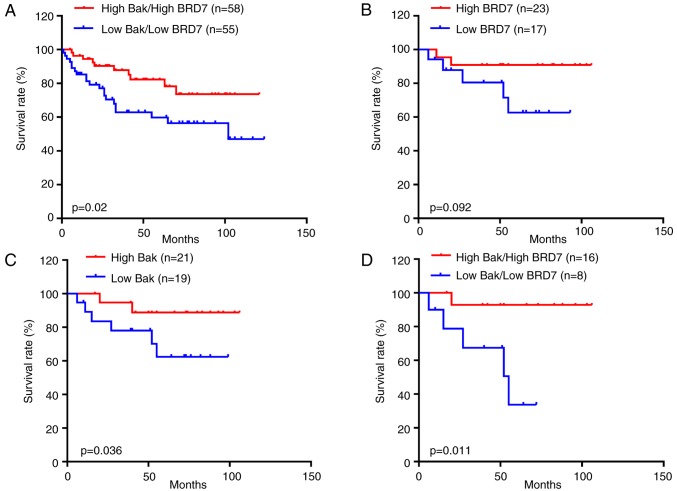
To investigate the role of BRD7 and Bak in breast cancer, the present study first evaluated the expression of BRD7 and Bak in breast cancer tissues. Bak was primarily expressed in the cytoplasm, while BRD7 was mainly expressed in the nucleus (Fig. 1A); their expressions were significantly decreased in breast cancer tissues (n=225) when compared with the normal control (n=62; Fig. 1B). In addition, the association between BRD7 and Bak was evaluated, and the protein levels of BRD7 were revealed to be positively correlated with Bak levels in breast cancer tissues (n=225, Pearson's r=0.4, P<0.001; Fig. 1C). Notably, the patients with low Bak and BRD7 expression had a lower survival rate than the patients with high Bak and BRD7 expression (P=0.02; Fig. 2A).
Figure 1.
Expression of BRD7 and Bak in breast cancer tissues. (A) Representative IHC images of BRD7 and Bak staining in normal breast and breast cancer tissues. Left-hand column, magnification, ×100; scale bar=100 µm; right-hand column, magnification, ×200; bar=50 µm. (B) Semi-quantification of BRD7 and Bak expression for IHC staining in normal breast and breast cancer tissues. (C) The correlation between BRD7 and Bak expression in breast cancer tissues. *P<0.05, as indicated. BRD7, bromodomain-containing protein 7; Bak, B-cell lymphoma 2 antagonist/killer; IHC, immunohistochemistry.
Figure 2.
Kaplan-Meier overall survival curves of patients with breast cancer and either high or low expression of Bak and BRD7 proteins. (A) Kaplan-Meier curves revealed that there were worse overall survival rates for breast cancer patients with low Bak/BRD7 expression when compared with patients with high Bak/BRD7 expression (P=0.02). (B) Breast cancer patients treated with paclitaxel with low BRD7 expression had lower overall survival rates than patients with high BRD7 expression, but the difference was not significant (P=0.092). (C) Breast cancer patients treated with paclitaxel with low Bak expression had poorer overall survival rates when compared with patients with high Bak expression (P=0.036). (D) Patients with high Bak/BRD7 expression had significantly higher overall survival rates when compared with patients with low Bak/BRD7 expression who were treated with paclitaxel (P=0.011). BRD7, bromodomain-containing protein 7; Bak, B-cell lymphoma 2 antagonist/killer.
Furthermore, to investigate the associations between Bak/BRD7 and paclitaxel treatment in breast cancer, 40 cases that had used therapeutic strategies including paclitaxel treatment were selected from the 225 patients with breast cancer. As a result, the patients treated with paclitaxel that had greater BRD7 expression had higher overall survival than those with low BRD7 expression; however, there was no statistically significant difference (P=0.092; Fig. 2B). While high Bak expression indicated a higher overall survival rate in patients treated with paclitaxel compared with those of patients with low Bak expression (P=0.036; Fig. 2C). In addition, the patients treated with paclitaxel with low Bak and BRD7 expression had a lower survival rate than those with high Bak and BRD7 expression (P=0.011; Fig. 2D).
BRD7 activates promoter activity and the expression of Bak in an indirect manner
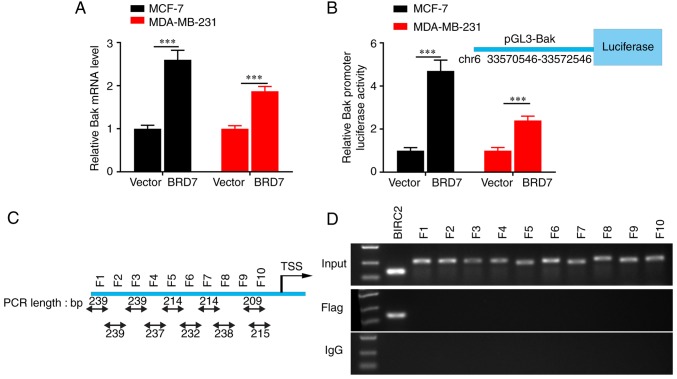
As chromatin remodeling function is crucial for BRD7-mediated transcriptional regulation (23) and BRD7 was positively correlated with Bak expression at the protein level in breast cancer tissues, the present study further investigated the effect and mechanism of BRD7 on Bak transcriptional regulation and expression. The results revealed that overexpression of BRD7 significantly upregulated the expression of Bak mRNA as detected by RT-qPCR assays (Fig. 3A), and increased the activity of the Bak promoter, as evaluated by dual-Luciferase reporter analysis (Fig. 3B). In addition, the ChIP assay was performed to investigate whether BRD7 binds to the Bak promoter. The potential promoter region was first predicted and then divided it into 10 fragments based on the DNA walking method (Fig. 3C). The results demonstrated that the Flag antibody couldn't pull down any of the fragments from the Bak promoter (Fig. 3D). These results indicated that BRD7 may control Bak expression at the transcriptional level in an indirect manner.
Figure 3.
BRD7 activates the Bak promoter in an indirect manner. (A) Reverse transcription-quantitative PCR was performed to measure the Bak mRNA levels following BRD7 expression. (B) Luciferase reporter assay was performed to measure the promoter activity of Bak following the expression of BRD7 in MCF-7 and MDA-MB-231 cells. (C) The schematic of the ChIP experiments. (D) ChIP was performed to determine the fragment of the Bak promoter that BRD7 binds to; BIRC2 was used as a positive control. ***P<0.001, as indicated. ChIP, chromatin immunoprecipitation; BRD7, bromodomain-containing protein 7; Bak, B-cell lymphoma 2 antagonist/killer; PCR, polymerase chain reaction; IgG, immunoglobulin G; F, fragment; TSS, transcription start site; BIRC2, baculoviral IAP repeat containing 2.
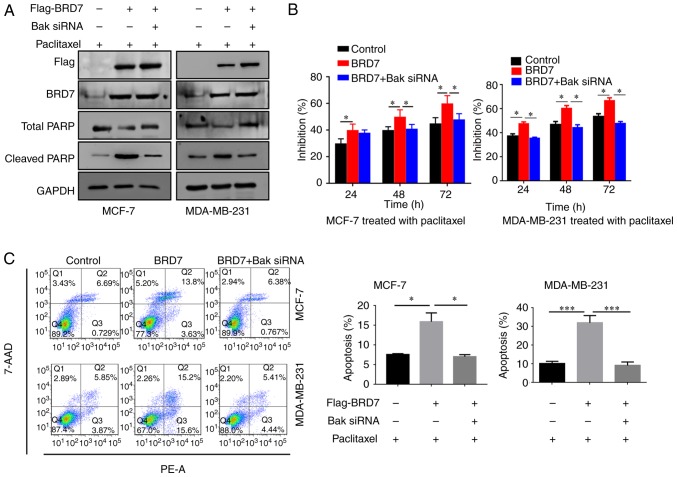
Silencing of Bak abolishes the BRD7-mediated inhibition of breast cancer cell proliferation
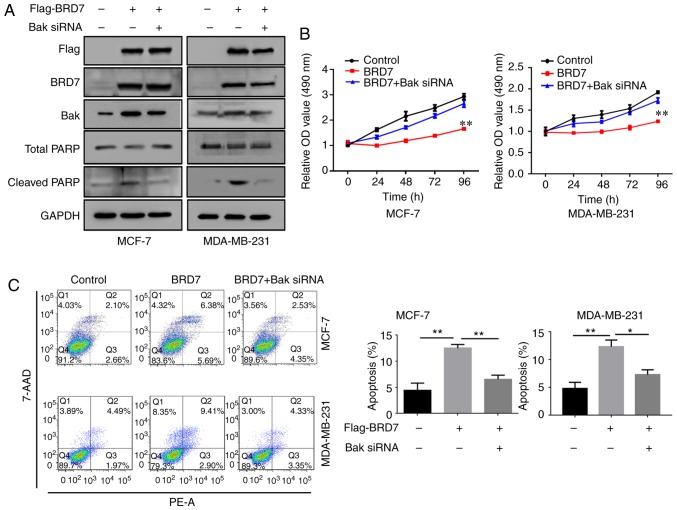
As BRD7 positively regulates the expression of Bak, the present study then investigated the effect of Bak on BRD7-mediated inhibition of proliferation. Therefore, BRD7 was overexpressed in the MCF-7 and MDA-MB-231 cells (Fig. 4A). Overexpression of BRD7 induced the expression of Bak as well as the apoptotic marker cleaved PARP; however, silencing of Bak by siRNA did not alter BRD7 expression (Fig. 4A). In addition, overexpression of BRD7 significantly inhibited cell proliferation in MCF-7 and MDA-MB-231 cells, which was abolished by Bak silencing (Fig. 4B). In addition, the promotive effects of BRD7 on cell apoptosis were also attenuated by Bak silencing (Fig. 4C).
Figure 4.
BRD7 inhibits breast cancer cell growth by activating Bak. (A) Western blotting analysis for Flag, BRD7, Bak, total PARP and cleaved PARP following the indicated treatments in MCF-7 and MDA-MB-231 cells. GAPDH was used as a loading control. (B) Cell Counting Kit-8 was performed to measure the cell viability of MCF-7 and MDA-MB-231 cells following the indicated treatments. **P<0.01 vs. Control. (C) Flow cytometry was conducted to measure the levels of cell apoptosis in MCF-7 and MDA-MB-231 cells following the indicated treatments. *P<0.05 and **P<0.01, as indicated. BRD7, bromodomain-containing protein 7; Bak, B-cell lymphoma 2 antagonist/killer; PARP, poly (adenosine diphosphate-ribose) polymerase; OD, optical density; 7-AAD, 7-aminoactinmycin D; PE, phycoerythrin; siRNA, small interfering RNA.
Silencing of Bak attenuates BRD7-enhanced paclitaxel cytotoxicity in breast cancer cells
As the expression of BRD7 and Bak was associated with the survival rate of patients treated with paclitaxel in breast cancer (Fig. 2D), the present study then detected the effect of Bak on BRD7-mediated paclitaxel cytotoxicity. As expected, overexpression of BRD7 enhanced the inhibitory effects of paclitaxel on cell proliferation and the promotion of cell apoptosis as supported by the significant increase in the rate of inhibition (Fig. 5B) and apoptosis (Fig. 5C) when compared with the negative control. Overexpression of BRD7 also increased the expression of cleaved PARP (Fig. 5A). However, silencing of Bak in cells expressing BRD7 decreased cleaved PARP expression, as well as the rate of inhibition (Fig. 5B) and apoptosis when compared with the BRD7 only group (Fig. 5C).
Figure 5.
Knockdown of Bak abolishes BRD7-enhanced chemosensitivity in breast cancer cells. (A) Western blotting analysis for Flag, BRD7, total PARP and cleaved PARP following the indicated treatments in MCF-7 and MDA-MB-231 cells. GAPDH was used as a loading control. (B) Cell Counting Kit-8 was performed to measure the cell viability of MCF-7 and MDA-MB-231 cells following the indicated treatments. (C) Flow cytometry was performed to measure cell apoptosis in MCF-7 and MDA-MB-231 cells following the indicated treatments. *P<0.05 and ***P<0.001, as indicated. BRD7, bromodomain-containing protein 7; Bak, B-cell lymphoma 2 antagonist/killer; PARP, poly (adenosine diphosphate-ribose) polymerase; siRNA, small interfering RNA; 7-AAD, 7-aminoactinmycin D; PE, phycoerythrin.
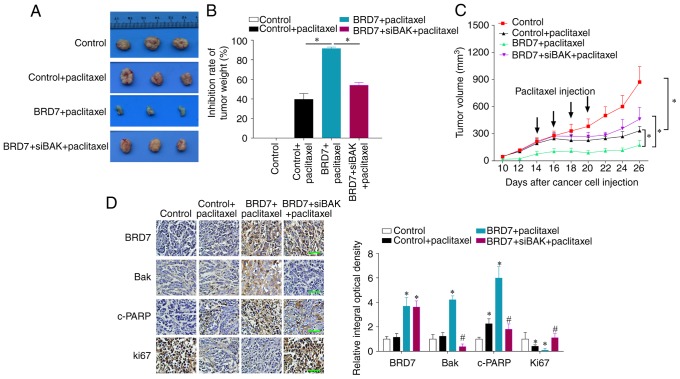
BRD7 enhances paclitaxel cytotoxicity by activating Bak in vivo
As BRD7 could inhibit cell proliferation and sensitize cancer cells to paclitaxel through Bak activation in breast cancer cells, the present study then chose to further validate the roles and mechanism of Bak in paclitaxel sensitization in vivo by tumor xenograft in nude mice. The results revealed that paclitaxel treatment suppressed tumor growth in vivo and BRD7 overexpression enhanced the inhibitory role of paclitaxel, while Bak silencing by siRNA reversed BRD7-mediated chemosensitivity as determined by evaluating tumor volume, tumor inhibition rate and tumor growth curves (Fig. 6A-C). The immunohistochemistry results demonstrated that BRD7 expression was significantly increased in BRD7 plus paclitaxel and BRD7 plus siBak and paclitaxel groups when compared with the control groups, and Bak expression was induced by BRD7 but silenced by siBak transfection (Fig. 6D). The expression of the apoptotic marker cleaved PARP was positively regulated by BRD7 and the expression of the proliferative marker Ki67 was negatively regulated by BRD7; while Bak silencing reversed the effect of BRD7 on the expression cleaved PARP and Ki67 (Fig. 6D). These results support the notion that BRD7 enhances paclitaxel cytotoxicity through the activation of Bak in vivo.
Figure 6.
Knockdown of Bak reverses BRD7-enhanced chemosensitivity in vivo. The MCF-7 cells transfected with BRD7 with or without Bak siRNA were injected into nude mice. On day 14 following cell injections, the mice were administered four paclitaxel injections, one every two days. (A) Mice were sacrificed and the tumors were obtained from mice on day 26 post-injections. (B) The tumor weight inhibition rate. *P<0.05, as indicated. (C) The tumor volumes were measured every 2-days. *P<0.05, as indicated. (D) The expression of BRD7, Bak, c-PARP and Ki67 in tumor sections was evaluated by immunohistochemistry. Magnification, ×400; Scale bars, 100 µm. *P<0.05 vs. control; #P<0.05 vs. BRD7+paclitaxel group. BRD7, bromodomain-containing protein 7; Bak, B-cell lymphoma 2 antagonist/killer; c-PARP, cleaved poly (adenosine diphosphate-ribose) polymerase; si-, small interfering RNA.
Discussion
The present study revealed that Bak was significantly decreased in breast cancer tissues when compared with the normal control. Our previous study demonstrated that low Bak expression was associated with a poorer prognostic outcome for patients with breast cancer (4). The present results revealed that patients with low Bak and BRD7 expression had lower survival rates than the patients with high Bak and BRD7 expression. Furthermore, greater Bak expression was associated with a higher overall survival rate in patients treated with paclitaxel compared with those of patients with low Bak expression. Our previous study demonstrated that Bak is a direct target of miR-125b, which mediated paclitaxel resistance in breast cancer cells (24,25), suggesting that Bak may be associated with drug resistance. In addition, decreased Bak expression was also observed in doxorubicin-resistant Ewing sarcoma cells (26), cisplatin-resistant ovarian cancer and non-small cell lung cancer (27,28), and docetaxel-resistant gastric cancer (29). Bak is a key effector for the apoptotic pathway; Bak autoactivation serves a key role in regulating the intrinsic apoptotic pathway in intact cells (30). Thus, activating Bak would be a promising strategy for triggering cancer cell apoptosis and overcoming drug-resistance. Some candidate anti-cancer drugs exhibit potent activity against multidrug-resistance by activating Bak, such as coumarin (31) and Leelamine in breast cancer (32). In addition, human Bak protein integrated in liposomes was designed to activate the mitochondrial apoptotic pathway in colon cancer cells and glioblastoma (33).
In the present study, BRD7 activated the Bak promoter and induced its expression, but it did not directly bind to its promoter. BRD7 is a multifunctional gene, exhibiting its functions through several pathways (6). As a tumor suppressor, BRD7 inhibited cancer cell growth via phosphoinositide 3-kinase (PI3K)/Akt, ER stress, ERK and hypoxia-inducible factor α (HIF1α)/lactate dehydrogenase A signaling (10,34–36). Furthermore, BRD7 regulated glucose homeostasis through glycogen synthase kinase 3β and X-box binding protein 1 (37,38). By binding to p53, BRD7 acetylates p53 in turn activating downstream targets (14,15). In the present study, ectopic expression of BRD7 in breast cancer cells inhibited cell proliferation, promoted apoptosis and sensitized cancer cells to paclitaxel, while Bak silencing abolished the BRD7-mediated inhibitory effects on breast cancer cell growth and paclitaxel sensitization. In line with these previous results, the present study also indicated that there may be a positive correlation between BRD7 and Bak expression in breast cancer tissues.
The present study also investigated BRD7 as a p53 co-activator and how it regulates Bak expression. Since MCF-7 contains wild-type p53 and MDA-MB-231 contains mutant p53, these two types of cancer cells were selected to investigate whether BRD7 regulation of Bak expression is p53 dependent. Previous studies have demonstrated that p53 upregulated modulator of apoptosis (PUMA), a target gene of p53, can directly activate proapoptotic Bak to permeabilize mitochondria, leading to caspase activation and apoptosis (39). Antagonizing the PI3K-AKT signaling pathway triggered PUMA promoter (40) and Bak release from myeloid cell leukemia sequence-1/Bcl-2/Bcl-xL, resulting in Bak activation and apoptosis (41). BRD7 interacts with p85α and facilitates the nuclear translocation of p85α to inhibit PI3K signaling (10,38). In addition, via the acetylation its promoter BRD7, as a co-activator of p53, can activate the downstream targets of p53, including Bak (42–44). These results suggest that BRD7 may activate Bak by regulating PI3K/Akt signaling, potentially in a p53-dependent and independent manner.
In conclusion, the present study demonstrated that BRD7 sensitizes breast cancer cells to paclitaxel by activating Bak, which may occur via an indirect pathway. These results support the function of BRD7 as a tumor suppressor and provide a novel mechanism by which BRD7 enhances chemotherapy in breast cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National Natural Science Foundation of China (grant nos. 81572748, 81772990 and 81802668), the Natural Science Foundation of Hunan Province (grant no. 2018JJ3776), the Fundamental Research Funds for the Central Universities of Central South University (grant nos. 2018zzts823 and 2018zzts233) and the Open-End Fund for the Valuable and Precision Instruments of Central South University (grant no. CSUZC201743).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
GL, RG, YLu and MZ made substantial contributions to the design of the study. JM, WN, XW, YZ, JG, HW and FL analyzed and interpreted the patient data. YLi, JG, WX, ZZ, SF, XL and XN performed the cell biological experiments. All authors contributed to writing the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethic Committee of Central South University (Changsha, China). All subjects provided written informed consent to participate in the present study.
Patient consent for publication
All patients provided written informed consent for the publication of the present study.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: An overview. Oncogene. 2008;27(Suppl 1):S2–S19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. BH3-only proteins: Orchestrators of apoptosis. Biochim Biophys Acta 1813. 2011:508–520. doi: 10.1016/j.bbamcr.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 3.Ghiotto F, Fais F, Bruno S. BH3-only proteins: The death-puppeteer's wires. Cytometry A. 2010;77:11–21. doi: 10.1002/cyto.a.20819. [DOI] [PubMed] [Google Scholar]
- 4.Luo Y, Wang X, Wang H, Xu Y, Wen Q, Fan S, Zhao R, Jiang S, Yang J, Liu Y, et al. High Bak expression is associated with a favorable prognosis in breast cancer and sensitizes breast cancer cells to paclitaxel. PLoS One. 2015;10:e138955. doi: 10.1371/journal.pone.0138955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birkinshaw RW, Czabotar PE. The BCL-2 family of proteins and mitochondrial outer membrane permeabilisation. Semin Cell Dev Biol. 2017;72:152–162. doi: 10.1016/j.semcdb.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Yu X, Li Z, Shen J. BRD7: A novel tumor suppressor gene in different cancers. Am J Transl Res. 2016;8:742–748. [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y, Zhao R, Wang H, Luo Y, Wang X, Niu W, Zhou Y, Wen Q, Fan S, Li X, et al. miR-141 is involved in BRD7-mediated cell proliferation and tumor formation through suppression of the PTEN/AKT pathway in nasopharyngeal carcinoma. Cell Death Dis. 2016;7:e2156. doi: 10.1038/cddis.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen CL, Wang Y, Pan QZ, Tang Y, Wang QJ, Pan K, Huang LX, He J, Zhao JJ, Jiang SS, et al. Bromodomain-containing protein 7 (BRD7) as a potential tumor suppressor in hepatocellular carcinoma. Oncotarget. 2016;7:16248–16261. doi: 10.18632/oncotarget.7637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Q, Wei L, Yang H, Yang W, Yang Q, Zhang Z, Wu K, Wu J. Bromodomain containing protein represses the Ras/Raf/MEK/ERK pathway to attenuate human hepatoma cell proliferation during HCV infection. Cancer Lett. 2016;371:107–116. doi: 10.1016/j.canlet.2015.11.027. [DOI] [PubMed] [Google Scholar]
- 10.Chiu YH, Lee JY, Cantley LC. BRD7, a tumor suppressor, interacts with p85α and regulates PI3K activity. Mol Cell. 2014;54:193–202. doi: 10.1016/j.molcel.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Y, Zhao R, Wei Y, Li M, Wang H, Niu W, Zhou Y, Qiu Y, Fan S, Zhan Y, et al. BRD7 expression and c-Myc activation forms a double-negative feedback loop that controls the cell proliferation and tumor growth of nasopharyngeal carcinoma by targeting oncogenic miR-141. J Exp Clin Cancer Res. 2018;37:64. doi: 10.1186/s13046-018-0734-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao R, Liu Y, Wang H, Yang J, Niu W, Fan S, Xiong W, Ma J, Li X, Phillips JB, et al. BRD7 plays an anti-inflammatory role during early acute inflammation by inhibiting activation of the NF-κB signaling pathway. Cell Mol Immunol. 2017;14:830–841. doi: 10.1038/cmi.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Zhao R, Guo C, Jiang S, Yang J, Xu Y, Liu Y, Fan L, Xiong W, Ma J, et al. Knockout of BRD7 results in impaired spermatogenesis and male infertility. Sci Rep. 2016;6:21776. doi: 10.1038/srep21776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burrows AE, Smogorzewska A, Elledge SJ. Polybromo-associated BRG1-associated factor components BRD7 and BAF180 are critical regulators of p53 required for induction of replicative senescence. Proc Natl Acad Sci USA. 2010;107:14280–14285. doi: 10.1073/pnas.1009559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drost J, Mantovani F, Tocco F, Elkon R, Comel A, Holstege H, Kerkhoven R, Jonkers J, Voorhoeve PM, Agami R, et al. BRD7 is a candidate tumour suppressor gene required for p53 function. Nat Cell Biol. 2010;12:380–389. doi: 10.1038/ncb2038. [DOI] [PubMed] [Google Scholar]
- 16.Harte MT, O'Brien GJ, Ryan NM, Gorski JJ, Savage KI, Crawford NT, Mullan PB, Harkin DP. BRD7, a subunit of SWI/SNF complexes, binds directly to BRCA1 and regulates BRCA1-dependent transcription. Cancer Res. 2010;70:2538–2547. doi: 10.1158/0008-5472.CAN-09-2089. [DOI] [PubMed] [Google Scholar]
- 17.Matissek KJ, Okal A, Mossalam M, Lim CS. Delivery of a monomeric p53 subdomain with mitochondrial targeting signals from pro-apoptotic Bak or Bax. Pharm Res. 2014;31:2503–2515. doi: 10.1007/s11095-014-1346-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J, Guo W, Zhou H, Luo N, Nie C, Zhao X, Yuan Z, Liu X, Wei Y. Mitochondrial p53 phosphorylation induces Bak-mediated and caspase-independent cell death. Oncotarget. 2015;6:17192–17205. doi: 10.18632/oncotarget.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieminen AI, Eskelinen VM, Haikala HM, Tervonen TA, Yan Y, Partanen JI, Klefström J. Myc-induced AMPK-phospho p53 pathway activates Bak to sensitize mitochondrial apoptosis. Proc Natl Acad Sci USA. 2013;110:E1839–E1848. doi: 10.1073/pnas.1208530110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Xu K, Xiong W, Zhou M, Wang H, Yang J, Li X, Chen P, Liao Q, Deng H, Li X, et al. Integrating ChIP-sequencing and digital gene expression profiling to identify BRD7 downstream genes and construct their regulating network. Mol Cell Biochem. 2016;411:57–71. doi: 10.1007/s11010-015-2568-y. [DOI] [PubMed] [Google Scholar]
- 22.Shapter FM, Waters DL. Genome walking. Methods Mol Biol 1099. 2014:133–146. doi: 10.1007/978-1-62703-715-0_12. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Zhou M, Luo X, Zhang L, Niu Z, Peng C, Ma J, Peng S, Zhou H, Xiang B, et al. Transcriptional regulation of BRD7 expression by Sp1 and c-Myc. BMC Mol Biol. 2008;9:111. doi: 10.1186/1471-2199-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou M, Liu Z, Zhao Y, Ding Y, Liu H, Xi Y, Xiong W, Li G, Lu J, Fodstad O, et al. MicroRNA-125b confers the resistance of breast cancer cells to paclitaxel through suppression of pro-apoptotic Bcl-2 antagonist killer 1 (Bak1) expression. J Biol Chem. 2010;285:21496–21507. doi: 10.1074/jbc.M109.083337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo Y, Wang X, Niu W, Wang H, Wen Q, Fan S, Zhao R, Li Z, Xiong W, Peng S, et al. Elevated microRNA-125b levels predict a worse prognosis in HER2-positive breast cancer patients. Oncol Lett. 2017;13:867–874. doi: 10.3892/ol.2016.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iida K, Fukushi J, Matsumoto Y, Oda Y, Takahashi Y, Fujiwara T, Fujiwara-Okada Y, Hatano M, Nabashima A, Kamura S, et al. miR-125b develops chemoresistance in Ewing sarcoma/primitive neuroectodermal tumor. Cancer Cell Int. 2013;13:21. doi: 10.1186/1475-2867-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dai Y, Jin S, Li X, Wang D. The involvement of Bcl-2 family proteins in AKT-regulated cell survival in cisplatin resistant epithelial ovarian cancer. Oncotarget. 2017;8:1354–1368. doi: 10.18632/oncotarget.13817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma J, Zhao Z, Wu K, Xu Z, Liu K. MCL-1 is the key target of adjuvant chemotherapy to reverse the cisplatin-resistance in NSCLC. Gene. 2016;587:147–154. doi: 10.1016/j.gene.2016.04.054. [DOI] [PubMed] [Google Scholar]
- 29.Kubo T, Kawano Y, Himuro N, Sugita S, Sato Y, Ishikawa K, Takada K, Murase K, Miyanishi K, Sato T, et al. BAK is a predictive and prognostic biomarker for the therapeutic effect of docetaxel treatment in patients with advanced gastric cancer. Gastric Cancer. 2016;19:827–838. doi: 10.1007/s10120-015-0557-1. [DOI] [PubMed] [Google Scholar]
- 30.Dai H, Ding H, Meng XW, Peterson KL, Schneider PA, Karp JE, Kaufmann SH. Constitutive BAK activation as a determinant of drug sensitivity in malignant lymphohematopoietic cells. Genes Dev. 2015;29:2140–2152. doi: 10.1101/gad.267997.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiang J, Wang Z, Liu Q, Li X, Sun J, Fung KP, Liu F. DMFC (3,5-dimethyl-7H-furo[3,2-g]chromen-7-one) regulates Bim to trigger Bax and Bak activation to suppress drug-resistant human hepatoma. Apoptosis. 2017;22:381–392. doi: 10.1007/s10495-016-1331-5. [DOI] [PubMed] [Google Scholar]
- 32.Sehrawat A, Kim SH, Hahm ER, Arlotti JA, Eiseman J, Shiva SS, Rigatti LH, Singh SV. Cancer-selective death of human breast cancer cells by leelamine is mediated by bax and bak activation. Mol Carcinog. 2017;56:337–348. doi: 10.1002/mc.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liguori L, Pastorino F, Rousset X, Alfano S, Cortes S, Emionite L, Daga A, Ponzoni M. Anti-tumor effects of Bak-proteoliposomes against glioblastoma. Molecules. 2015;20:15893–15909. doi: 10.3390/molecules200915893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Y, Wang B, Gao S. BRD7 Acts as a tumor suppressor gene in lung adenocarcinoma. PLoS One. 2016;11:e156701. doi: 10.1371/journal.pone.0156701. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Li D, Yang Y, Zhu G, Liu X, Zhao M, Li X, Yang Q. MicroRNA-410 promotes cell proliferation by targeting BRD7 in non-small cell lung cancer. FEBS Lett. 2015;589:2218–2223. doi: 10.1016/j.febslet.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 36.Niu W, Luo Y, Wang X, Zhou Y, Li H, Wang H, Fu Y, Liu S, Yin S, Li J, et al. BRD7 inhibits the Warburg effect and tumor progression through inactivation of HIF1α/LDHA axis in breast cancer. Cell Death Dis. 2018;9:519. doi: 10.1038/s41419-018-0536-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Golick L, Han Y, Kim Y, Park SW. BRD7 regulates the insulin-signaling pathway by increasing phosphorylation of GSK3β. Cell Mol Life Sci. 2018;75:1857–1869. doi: 10.1007/s00018-017-2711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park SW, Herrema H, Salazar M, Cakir I, Cabi S, Basibuyuk Sahin F, Chiu YH, Cantley LC, Ozcan U. BRD7 regulates XBP1s' activity and glucose homeostasis through its interaction with the regulatory subunits of PI3K. Cell Metab. 2014;20:73–84. doi: 10.1016/j.cmet.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang L, Li A, Liao G, Yang F, Yang J, Chen X, Jiang X. Curcumol triggers apoptosis of p53 mutant triple-negative human breast cancer MDA-MB 231 cells via activation of p73 and PUMA. Oncol Lett. 2017;14:1080–1088. doi: 10.3892/ol.2017.6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bean GR, Ganesan YT, Dong Y, Takeda S, Liu H, Chan PM, Huang Y, Chodosh LA, Zambetti GP, Hsieh JJ, et al. PUMA and BIM are required for oncogene inactivation-induced apoptosis. Sci Signal. 2013;6:ra20. doi: 10.1126/scisignal.2003483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahmani M, Aust MM, Attkisson E, Williams DJ, Jr, Ferreira-Gonzalez A, Grant S. Dual inhibition of Bcl-2 and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in human myeloid leukemia cells through a GSK3- and Bim-dependent mechanism. Cancer Res. 2013;73:1340–1351. doi: 10.1158/0008-5472.CAN-12-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Huang K, O'Neill KL, Pang X, Luo X. Bax/Bak activation in the absence of Bid, Bim, Puma, and p53. Cell Death Dis. 2016;7:e2266. doi: 10.1038/cddis.2016.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones NA, Turner J, McIlwrath AJ, Brown R, Dive C. Cisplatin- and paclitaxel-induced apoptosis of ovarian carcinoma cells and the relationship between bax and bak up-regulation and the functional status of p53. Mol Pharmacol. 1998;53:819–826. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.