Abstract
As small non-coding RNA molecules, microRNAs (miRs) function in the regulation of tumorigenesis. Proliferation in ovarian cancer is considered to be associated with miR-16; however, the role of miR-16 in the migration and invasion of ovarian cancer cells remains unclear. The results of the present study demonstrated that miR-16 expression is downregulated in the ovarian cancer SKOV3 and OVCAR3 cell lines compared with that in normal ovarian epithelial cells (OECs). miR-16 overexpression inhibited the proliferation, migration and invasion of SKOV3 and OVCAR3 cells, and decreased the expression of matrix metallopeptidase (MMP)2 and MMP9. Additionally, miR-16 upregulated the expression of cadherin 1, an intercellular adhesion molecule, and downregulated the expression of some mesenchymal markers, including snail family transcriptional repressor 2, snail family transcriptional repressor 1, Vimentin, twist family BHLH transcription factor 1 and cadherin 2 in SKOV3 and OVCAR3 cells. Furthermore, it was indicated that miR-16 overexpression in SKOV3 and OVCAR3 cells resulted in a significant increase in anti-glycogen synthase kinase 3 β expression and a decrease in the expression of Wnt family member 3A, β-catenin, MYC proto-oncogene, BHLH transcription factor and cyclin D1 compared with the NC group. The results of the present study indicated that miR-16 exerts a suppressive effect on cell migration and invasion in ovarian cancer in vitro, through inactivation of the Wnt/β-catenin signaling pathway. The data suggest that miR-16 may be a potential therapeutic agent for the treatment and prevention of ovarian cancer.
Keywords: microRNA-16, ovarian cancer, Wnt family member 3A, migration, invasion
Introduction
One of the most common solid tumors is ovarian carcinoma. In the United States, ovarian cancer was the fifth leading cause of cancer-associated mortality worldwide in 2014 (1). As a result of limited screening and delayed diagnosis, the majority of patients present with advanced-stage disease at the time of diagnosis, with a 5-year survival rate of ~20% (2). The poor prognosis of ovarian cancer is mainly caused by tumor metastasis or recurrence (3,4). Therefore, the development of effective therapeutic strategies and targets for the control of ovarian cancer metastasis is crucial.
MicroRNAs (miRs/miRNAs) consist of 21–23 endogenous non-coding nucleotides that control gene expression by binding to the 3′-untranslated region of their target genes. This binding results in the suppression of messenger (m)RNA translation or the degradation of mRNA. Emerging evidence has demonstrated that the development and progression of ovarian cancer involve the deregulation of various miRNAs, suggesting that miRNAs could be novel diagnostic and therapeutic markers (2,5–7). For example, miR-205 has been reported to induce cell invasion by repressing transcription factor 21 in human ovarian cancer (8). In addition, miR-212 serves a tumor-suppressing role in ovarian cancer cells by inhibiting the expression of heparin-binding EGF-like growth factor (9).
miR-16 was the first deregulated miRNA reported to function as a tumor suppressor in a number of types of cancer, including B-cell chronic lymphocytic leukemia and pituitary adenomas (10,11). In addition, miR-16 was reported to be downregulated in numerous tumor types, including chronic lymphocytic leukemia, prostate cancer and pituitary adenomas (12). The reduced expression of miR-16 was also been reported in ovarian cancer tissues and in a number ovarian cancer cell lines, including OV-202, CP70 and A2780 (13). miR-16 overexpression markedly inhibits the proliferation and clonal growth of ovarian cancer cells, indicating the involvement of miR-16 in the carcinogenesis of ovarian cancer via the deregulation of proliferation (13). However, the role and underlying mechanism of miR-16 in ovarian cancer cell migration and/or invasion requires further investigation.
The objective of the present study was to investigate the functions and underlying mechanisms of miR-16 in ovarian cancer. Using SKOV3 and OVCAR3 cells as a model, the effects of miR-16 on proliferation, migration and invasion were studied, in addition to the epithelial-mesenchymal transition (EMT) of ovarian cancer in vitro. The present study aimed to provide novel insights to improve the treatment and prevention of ovarian cancer.
Materials and methods
Cell lines
Human ovarian cancer cell lines, SKOV3 and OVCAR3, were obtained from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Human ovarian epithelial cells (OECs; cat. no. 7310) were purchased from ScienCell Research Laboratories, Inc. (San Diego, CA, USA). The cell lines were cultured in RPMI-1640 medium (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1% antibiotic-antimycotic solution (100 U/ml penicillin and 100 µg/ml streptomycin). The OECs were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS. All cells were cultured at 37°C with 5% CO2. SKOV3 and OVCAR3 cells were used in all the following experiments.
Reagents
CCK-8 solution was obtained from Abcam (Cambridge, UK). The following antibodies were purchased from Abcam: Rabbit anti-matrix metallopeptidase (MMP)2, anti-MMP9, anti-cadherin 1 (E-CAD), anti-cadherin 2 (N-CAD), anti-MYC, anti-Cyclin D1, anti-Vimentin, anti-Slug, anti-snail family transcriptional repressor 1 (SNAIL), anti-twist family BHLH transcription factor 1 (TWIST), anti-GAPDH, anti-Wnt family member 3A (Wnt3a), anti-glycogen synthase kinase 3 β (Gsk3β) and anti-β-catenin. Goat anti-rabbit and anti-mouse antibodies labeled with horseradish peroxidase were purchased from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA). Transwell chambers were purchased from Corning, Inc. (Corning, NY, USA).
miRNA synthesis, preparation and storage
The synthetic miR-16 mimic and the mimic negative control (NC) were purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The synthetic samples were first centrifuged at 12,000 × g for 20 min at 25°C following dilution with 250 µl diethyl-pyrocarbonate-treated water as the mother liquor per 5 nmol miRNA. The samples were subpackaged and stored at −20°C until further use. The miR sequences were as follows: miR-16 mimic, 5′-UAGCAGCACGUAAUAUUGGCGCCAAUAUUUACGUGCUGCUAUU-3′; and miR-16 mimic NC, 5′-UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT-3′.
Transfection of cells with miRNAs
Cells were inoculated 1 day prior to transfection in RPMI-1640 medium without penicillin-streptomycin solution in 6-well plates, (2,000 µl per well) with an increase or decrease in the proportion of cells per well. Upon reaching a confluence of 30–50%, the cells were transfected. Opti-MEM (250 µl) was added to dilute the miRNAs (50 nmol) and Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) was subsequently diluted separately in Opti-MEM (250 µl) by blending gently. This mixture was incubated for 5 min at room temperature and gently mixed with the diluted miRNAs prior to further incubation at room temperature for 20–30 min. The miRNA/Lipofectamine 2000 compound was added to the 6-well plates, which were subsequently gently agitated. After 6 h, RPMI-1640 medium containing 10% FBS was added, and the cells were cultivated at 37°C in a CO2 incubator for 24–72 h.
Cell Counting Kit (CCK)-8 assay
A CCK-8 assay was used to assess proliferation. Cells were seeded into 96-well plates (Corning, Inc.) and transfected with either the miR-16 mimic or the NC as aforementioned. CCK-8 (10 µg) solution (Dojindo Molecular Technologies, Inc., Kumamoto, Japan) was added into 100 µl RPMI-1640 medium at 24, 48 and 72 h post-transfection. The cells were diluted with CCK-8 in RPMI-1640 medium for 2 h, lysed in radioimmunoprecipitation assay (RIPA) buffer and centrifuged at 13,000 × g for 10 min at 4°C. The absorbance of the supernatants was measured at a wavelength of 450 nm on an iMark microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RNA samples were qualified by using a UV spectrophotometer, and optical density (OD)260/OD280 was limited to 1.8–2.0. Total RNA was reverse transcribed to cDNA using a reverse transcription system (cat. no. 4368814; Fermentas, Thermo Fisher Scientific, Inc.). In brief, total RNA was incubated at 70°C for 10 min and centrifuged at 1,000 × g for 20 min at room temperature. cDNA was synthesized by Avian Myeloblastosis Virus Reverse Transcriptase (Takara Bio, Inc., Otsu, Japan), according to the manufacturer's protocols. RT-qPCR with SYBR Green qPCR dye (Toyobo Life Science, Osaka, Japan) was conducted, according to the manufacturer's protocol, to study the quantitative expression of miR-16. The thermocycling conditions were as follows: 45°C for 5 min for reverse transcription, followed by 94°C for 30 sec and then 40 cycles of 94°C for 5 sec and 60°C for 30 sec. Three replicates were included in the RT-qPCR analysis. The relative expression levels of miR-16 were normalized to β-actin and calculated using the 2−∆∆Cq method (14). The sequences of the primers used are: miR-16, 5′-TAGCAGCACGTAAATATTGGCG-3′ (forward) and 5′-TGCGTGTCGTGGAGTC-3′ (reverse); and GAPDH, 5′-AGAAGGCTGGGGCTCATTTG-3′ (forward) and 5′-AGGGGCCATCCACAGTCTTC-3′ (reverse).
Scratch assay
Overall, ~1×105 cells were seeded into 12-well plates (Costar; Corning, Inc.) and incubated at 37°C until reaching ≥90% confluency. The tip of a sterile 10-µl pipette was used to create a scratch at time 0 h for each experiment. After 48 h, an inverted microscope was used to image the cells. The migration distance was calculated as follows: Distance=(gap width at 0 h-gap width at 48 h)/gap width at 0 h.
Invasion assay
For the invasion assays, 24-well Transwell chamber inserts with an 8-µm pore size (BD Biosciences, Franklin Lakes, NJ, USA) were used. In total, ~1×105 cells/well were resuspended in 200 µl FBS-free medium and seeded on the upper chamber, which featured a Matrigel-coated membrane. Additionally, 500 µl RPMI-1640 medium supplemented with 10% FBS was added to the lower chamber, and the plates were incubated for 24 h at 37°C with 5% CO2. Finally, the membranes were stained with 0.1% crystal violet for 10 min at room temperature. An inverted microscope was used to count the number of cells on each membrane. Each assay was repeated at least three times.
Western blot analysis
RIPA lysis buffer was applied to the protein extracts from the SKOV3 and OVCAR3 cells, according to the manufacturer's protocol. Bovine serum albumin was used as the standard for determining the protein concentration by the Bradford protein assay (Bio-Rad Laboratories, Inc.). The expression levels of E-CAD, N-CAD, Vimentin, Slug, Twist, SNAIL, GAPDH, MMP2, MMP9, Wnt3a, Gsk3β, β-catenin, MYC proto-oncogene, BHLH transcription factor (c-MYC) and cyclin D1 were determined by western blot analysis. Equal amounts (20 µg) of lysate were subjected to 10% SDS-PAGE, and the proteins were subsequently transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA, USA) using a semidry transfer method. The PVDF membrane was blocked with 5% skimmed milk in Tween 20-BST buffer for 2 h at room temperature. Following blocking, the membrane was incubated overnight with the primary antibodies at 4°C followed by incubation for 1 h with a suitable secondary antibody at room temperature. An Enhanced Chemiluminescence Detection kit (Thermo Fisher Scientific, Inc.) was used for the detection of proteins, according to the manufacturer's protocols. Protein bands were subsequently detected using a Bio-Rad imaging system (Bio-Rad Laboratories, Inc.), and band intensity was quantified by densitometry analysis using Image-Pro Plus 4.5 software (Media Cybernetics, Inc., Rockville, MD, USA). The primary antibodies against MMP9 (cat. no. ab38898; 1:1,000), MMP2 (cat. no. ab37150; 1:1,000), GAPDH (cat. no. ab8245; 1:10,000), TWIST (cat. no. ab50581; 1:1,000), Vimentin (cat. no. ab8978; 1:1,000), SNAIL (cat. no. ab53519; 1:1,000), Slug (cat. no. ab27568; 1:1,000), E-CAD (cat. no. ab40772; 1:1,000), N-CAD (cat. no. ab18203; 1:1,000), β-catenin (cat. no. ab16051; 1:1,000), Gsk3β (cat. no. ab32391; 1:1,000), Wnt3a (cat. no. ab219412; 1:1,000), c-MYC (cat. no. ab32072; 1:1,000) and cyclin D1 (cat. no. ab16663; 1:1,000) were purchased Abcam. Goat anti-rabbit (cat. no. sc-2004; 1:10,000) and anti-mouse antibodies (cat. no. sc-2005; 1:10,000) labeled with horseradish peroxidase were purchased from Santa Cruz Biotechnology, Inc.
Statistical analysis
The SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA) was used for the conduction of all statistical tests. The data were compared by one-way analysis of variance followed by Dunnett's multiple comparison test. All data are expressed as the mean ± standard deviation. All the experiments were repeated in triplicate. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of miR-16 in human ovarian cancer cell lines
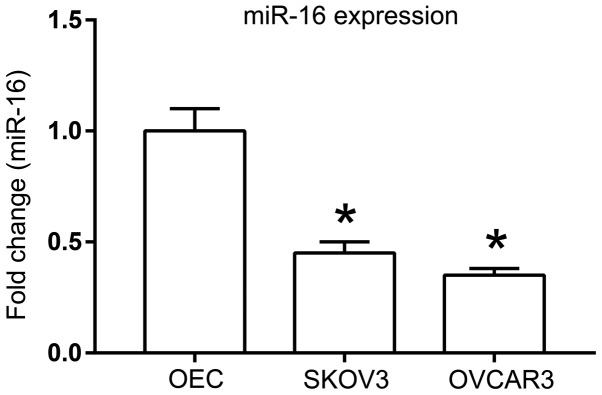
A previous study identified that miR-16 expression is downregulated in a number of ovarian cancer cell lines, including OV-167, CP-70, A2780 and OV-202 (13). To further investigate the role of miR-16 in other ovarian cancer cell lines, SKOV3 and OVCAR3, the expression of miR-16 was first examined with RT-qPCR. Significant downregulation of miR-16 expression was observed in the SKOV3 and OVCAR3 cells compared with that in the ovarian epithelial cells (P<0.05; Fig. 1).
Figure 1.
miR-16 expression in SKOV3 and OVCAR3 ovarian cancer cells compared with normal OEC cells. *P<0.05 compared with OEC. OEC, normal ovarian epithelial cells, miR, microRNA.
Effect of miR-16 on the proliferation of human ovarian cancer cells
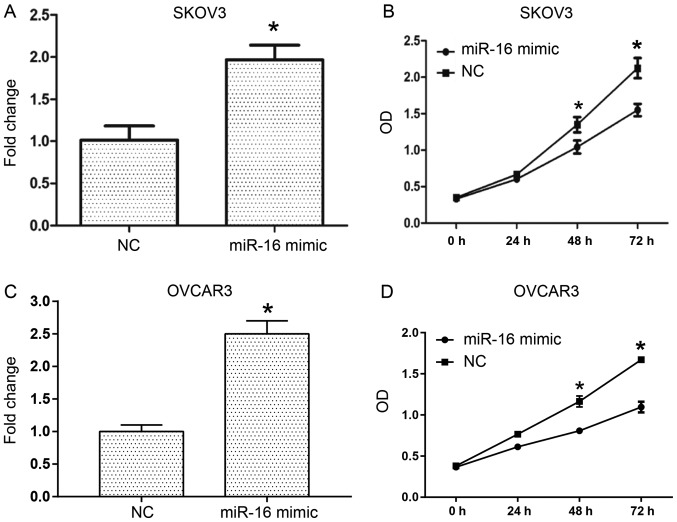
To investigate the role of miR-16 in the proliferation of human ovarian cancer cells, transient transfection of miR-16 mimic and NC was conducted into SKOV3 and OVCAR3 cells (Fig. 2). RT-qPCR was performed to examine the miR-16 expression level in the SKOV3 and OVCAR3 cells (Fig. 2A and C), and the proliferative ability of the cells was assessed with a CCK-8 assay. The results indicated that following transfection for 3 days, miR-16 overexpression in the SKOV3 and OVCAR3 cells significantly decreased the OD values compared with the NC (Fig. 2B and D).
Figure 2.
miR-16 inhibits the proliferation of ovarian cancer cells. (A) The inhibition efficiency of miR-16 mimics with regard to the expression of miR-16 in SKOV3 cells. (B) The proliferation of ovarian cancer in SKOV3 cells between 0 and 72 h. (C) The inhibition efficiency of miR-16 mimics with regard to the expression of miR-16 in OVCAR3 cells. (D) The proliferation of ovarian cancer in OVCAR3 cells between 0 and 72 h. *P<0.05 compared with the NC. OD, optical density; miR, microRNA; NC, negative control.
Effect of miR-16 on the migration and invasion of SKOV3 cells
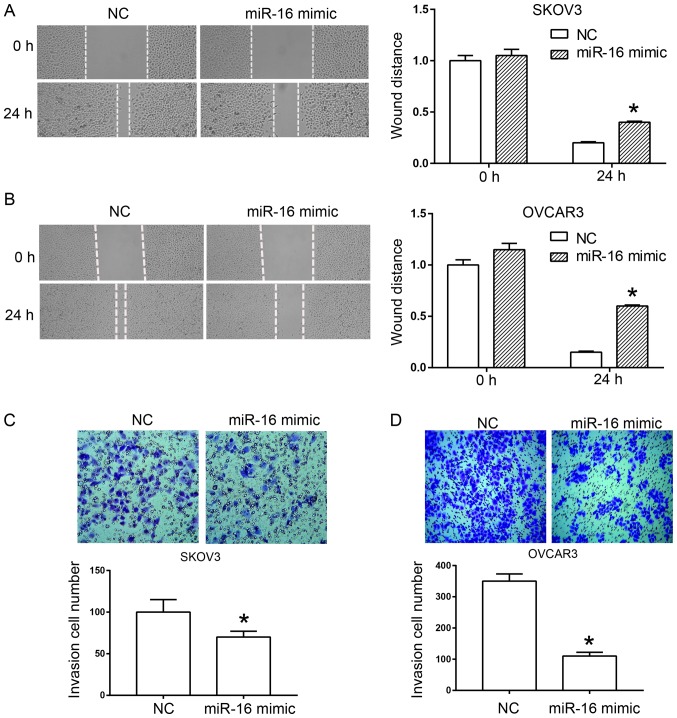
A scratch test and a Transwell assay were used to determine the effect of miR-16 on the migration and invasion of human ovarian cancer cells, respectively. In the scratch test, miR-16 overexpression significantly inhibited wound healing of the SKOV3 and OVCAR3 cells compared with the NC (Fig. 3A and B). In the Transwell assay, a significant reduction was observed in the invasion rate of SKOV3 and OVCAR3 cells compared with the NC due to the overexpression of miR-16 (Fig. 3C and D).
Figure 3.
miR-16 inhibits the migration and invasion of ovarian cancer cells. miR-16 inhibited the migration of (A) SKOV3 cells and (B) OVCAR3 cells. scratch assay: magnification, ×40. miR-16 suppressed the invasion of (C) SKOV3 and (D) OVCAR3 cells in a Transwell chamber invasion assay. Transwell chamber invasion assay: magnification, ×100. *P<0.05 compared with the NC. miR, microRNA; NC, negative control.
Effect of miR-16 on MMP expression in human ovarian cancer cells
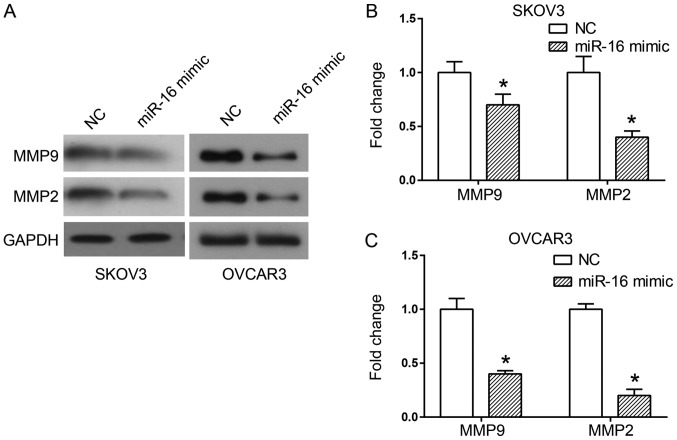
Two critical proteins involved in cell migration and invasion are MMP2 and MMP9 (15). A western blot analysis was used to detect the expression of MMP proteins when miR-16 was overexpressed, in order to observe the association between these proteins and the invasion and migration of miR-16-regulated cells. The results indicated a significant decrease in MMP2 and MMP9 expression levels in the cells transfected with the miR-16 mimic in comparison with the cells transfected with the NC (P<0.05; Fig. 4).
Figure 4.
Effect of miR-16 on the expression level of MMP proteins in SKOV3 and OVCAR3 cells. (A) The expression of MMP9 and MMP2 in SKOV3 and OVCAR3 cells. Quantification of protein bands in (B) SKOV3 and (C) OVCAR3 cells (n=5). *P<0.05 compared with the NC. MMP, matrix metallopeptidase; miR, microRNA; NC, negative control.
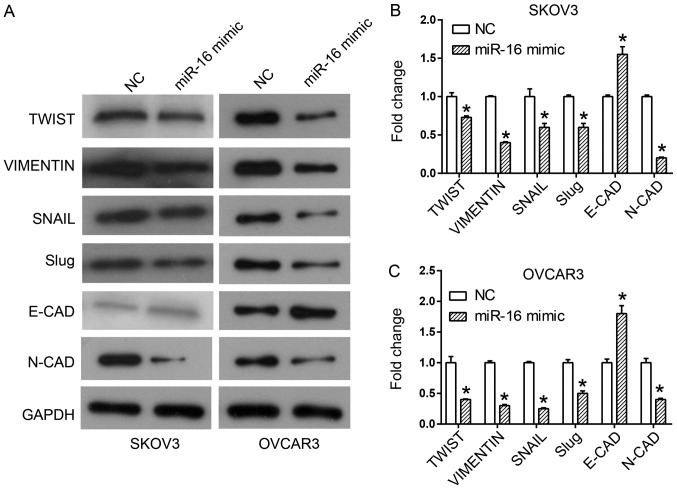
Effect of miR-16 on the expression of EMT-associated proteins in human ovarian cancer cells
EMT is an important process by which epithelial cells gain migratory and invasive capabilities by transforming into mesenchymal stem cells (16,17). Western blot analysis was utilized to observe the expression of different EMT markers upon miR-16 overexpression, in order to investigate whether EMT mediated the effect of miR-16 on migration and invasion. Overexpression of miR-16 upregulated the expression of the epithelial marker, E-CAD, and downregulated the expression of the mesenchymal markers, including N-CAD, Slug, SNAIL, Vimentin and Twist in the SKOV3 and OVCAR3 cells (Fig. 5).
Figure 5.
Effect of miR-16 on the expression level of EMT-associated proteins in SKOV3 and OVCAR3 cells. (A) Expression of EMT-associated proteins, including Twist, Vimentin, SNAIL, Slug, E-CAD and N-CAD in SKOV3 and OVCAR3 cells. Quantification of expression levels of EMT-associated proteins in (B) SKOV3 and (C) OVCAR3 cells *P<0.05 compared with the NC. Slug, Snail family transcriptional repressor 2; SNAIL, snail family transcriptional repressor 1; Twist, twist family BHLH transcription factor 1, E-CAD, cadherin 1; N-CAD, cadherin 2; miR, microRNA; NC, negative control; EMT, epithelial-mesenchymal transition.
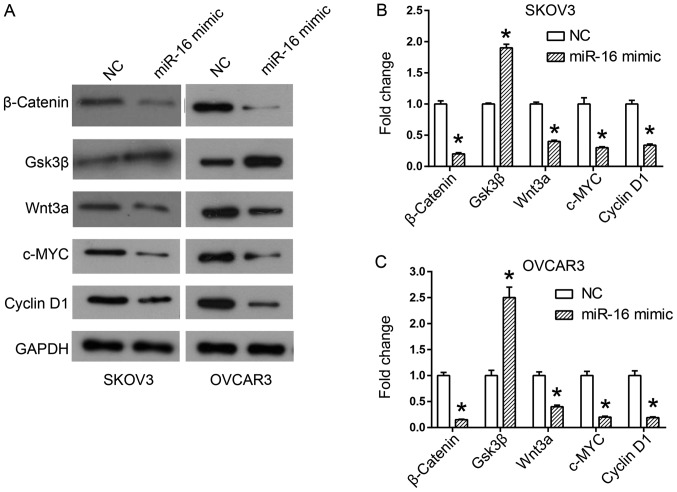
Effect of miR-16 on the Wnt/β-catenin signaling pathway in human ovarian cancer cells
The results of previous studies have demonstrated the important role of Wnt/β-catenin signaling in the acquisition of the EMT phenotype, and in cancer metastasis (18,19). Therefore, the expression of proteins involved in the Wnt/β-catenin pathway was examined in miR-16-regulated SKOV3 and OVCAR3 cells by western blot analysis. The results of the western blot analysis revealed an increase in Gsk3β expression, but a decrease in Wnt3a and β-catenin expression in the miR-16 mimic-transfected cells (Fig. 6). In addition, the downstream members of the Wnt/β-catenin pathway, c-MYC and cyclin D1, were additionally downregulated by the miR-16 mimic (Fig. 6).
Figure 6.
Effect of miR-16 on the expression of Wnt/β-catenin signaling pathway-associated proteins in SKOV3 and OVCAR3 cells. (A) Expression of Wnt/β-catenin signaling pathway components, including β-catenin, Gsk3β, Wnt3a, c-MYC and cyclin D1 in SKOV3 and OVCAR3 cells. Quantification of expression levels of Wnt/β-catenin signaling pathway components in (B) SKOV3 and (C) OVCAR3 cells. *P<0.05 compared with the NC. Gsk3β, glycogen synthase kinase 3 beta; c-MYC, MYC proto-oncogene, BHLH transcription factor; Wnt3a, Wnt family member 3A; miR, microRNA; NC, negative control.
Discussion
An miR-15a/miR-16-1 cluster was identified as a tumor suppressor in chronic lymphocytic leukemia, providing the first evidence linking miRNAs to human cancer (13). Following this pioneering study, there has been increasing evidence of the significant role of miRNAs in the early diagnosis, prognosis, prevention and therapeutic evaluation of cancer (2,20–22). Identification of alterations in specific miRNAs, in addition to understanding their functions in each type of cancer will improve the accuracy of diagnosis and prognosis of numerous types of cancer. Previous studies have indicated that certain miRNAs, including the members of the miR-200 and let-7 families, are involved in the progression and prognosis of ovarian cancer, as well as the response of ovarian cancer to chemotherapy (23). Bhattacharya et al (13) reported that miR-16 is downregulated in ovarian cancer cell lines and patient samples, whereas miR-16 upregulation inhibits the proliferation of ovarian carcinoma cells (13). The specific roles and underlying mechanisms of miR-16 in ovarian tumor metastasis remain unclear.
In the present study, downregulation of miR-16 expression was observed in two other ovarian cancer cell lines, SKOV3 and OVCAR3, providing evidence of the role of miR-16 in the process of proliferation. The results of the present study are consistent with those in the study by Bhattacharya et al (13), where miR-16 was downregulated in ovarian cancer cell lines and tumor tissues. Additionally, the present study indicated that overexpression of miR-16 inhibited the migration and invasion of SKOV3 and OVCAR3 cells. Previous studies have also examined the roles of miR-16 in tumor metastasis. Li et al (24) reported that miR-16-1 functions as a negative regulator of cell migration and invasion in glioma. In addition, Wu et al (25) revealed the suppressive effects of miR-16 on the metastasis of hepatocellular carcinoma.
In addition, the results of the present study indicate that the overexpression of miR-16 is able to decrease MMP2 and MMP9 expression in human ovarian cancer cells. The MMPs are a group of proteases involved in the degradation of the extracellular matrix, as these proteases also regulate invasion and tumor metastasis (15,17,18). MMP2 expression levels have been reported to be increased in patients with ovarian cancer with advanced tumors and metastasis (26). Therefore, the roles of miR-16 in ovarian cancer invasion and migration may be associated with the effect of MMPs on the extracellular matrix.
In addition, the effect of miR-16 on EMT may be another factor that regulates tumor metastasis. During EMT, epithelial cells lose their apical-basal polarity and cell-cell adhesion, allowing their conversion to motile mesenchymal cells, as this conversion promotes invasion and metastasis in cancer (16,17,27,28). Decreased expression of the intercellular adhesion molecule E-CAD has been reported as an EMT marker, along with increased expression of a series of mesenchymal markers, including Slug, SNAIL and Vimentin (27,29). In a previous study, Wang et al (30) reported that miR-16 may inhibit the expression of EMT-associated proteins in human glioma. Wang et al (31) reported that miR-16 mimics inhibited transforming growth factor-β1-induced EMT via activation of autophagy in non-small cell lung carcinoma cells. In the present study, it was determined that overexpression of miR-16 in SKOV3 and OVCAR3 cells inhibited the expression of Slug, SNAIL, Vimentin and Twist, but downregulated the expression of E-CAD.
It was also revealed that miR-16 mimics led to a significant increase in the expression of Gsk3β and decreased the expression levels of Wnt3a and β-catenin in SKOV3 and OVCAR3 cells. The Wnt/β-catenin signaling pathway has a regulatory function in a number of cellular processes, including development, differentiation, proliferation and adult tissue homeostasis. Therefore, aberrant Wnt/β-catenin signaling has been indicated to be involved in the pathogenesis of multiple tumors (18,19,32,33). Additional evidence indicates that the activation of the Wnt/β-catenin signaling pathway promotes the EMT process and the secretion of MMP2 in cancer cells, providing them with an increased ability for survival and metastasis (18,34–36).
However, the present study included a number of limitations. Firstly, the study was conducted to examine the effects of miR-16 on SKOV3 and OVCAR3 cell proliferation, invasion and metastasis, and to determine the involved signaling pathways. However, the target genes of miR-16 in these processes remain unknown. Further experiments are required. Secondly, this study is an in vitro study; therefore, in vivo studies should be conducted in the future to confirm the findings.
In conclusion, the present study demonstrated that miR-16 served a negative role in the proliferation, invasion and metastasis of SKOV3 and OVCAR3 cells. Furthermore, overexpression of miR-16 inhibited the process of EMT by inactivating the Wnt/β-catenin signaling pathway. The aforementioned results suggest that miR-16 may be a promising therapeutic target for ovarian cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
XW contributed to the study design, data acquisition and analysis, and drafted the manuscript. NL was involved in the study design, data acquisition and revision of the manuscript. LY and YS assisted in the performance of the statistical analysis. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Prahm KP, Novotny GW, Høgdall C, Høgdall E. Current status on microRNAs as biomarkers for ovarian cancer. APMIS. 2016;124:337–355. doi: 10.1111/apm.12514. [DOI] [PubMed] [Google Scholar]
- 3.Yeung TL, Leung CS, Yip KP, Au Yeung CL, Wong ST, Mok SC. Cellular and molecular processes in ovarian cancer metastasis. A review in the theme: Cell and molecular processes in cancer metastasis. Am J Physiol Cell Physiol. 2015;309:C444–C456. doi: 10.1152/ajpcell.00188.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Y, Kim S, Kim IM. Regulation of metastasis by microRNAs in ovarian cancer. Front Oncol. 2014;4:143. doi: 10.3389/fonc.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Nadeem L, Connor K, Xu G. Mechanisms and therapeutic targets of microRNA-associated chemoresistance in epithelial ovarian cancer. Curr Cancer Drug Targets. 2016;16:429–441. doi: 10.2174/1568009616666160404121105. [DOI] [PubMed] [Google Scholar]
- 6.Wang ZH, Xu CJ. Research progress of MicroRNA in early detection of ovarian cancer. Chin Med J (Engl) 2015;128:3363–3370. doi: 10.4103/0366-6999.171459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langhe R. microRNA and ovarian cancer. Adv Exp Med Biol. 2015;889:119–151. doi: 10.1007/978-3-319-23730-5_8. [DOI] [PubMed] [Google Scholar]
- 8.Wei J, Zhang L, Li J, Zhu S, Tai M, Mason CW, Chapman JA, Reynolds EA, Weiner CP, Zhou HH. MicroRNA-205 promotes cell invasion by repressing TCF21 in human ovarian cancer. J Ovarian Res. 2017;10:33. doi: 10.1186/s13048-017-0328-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei LQ, Liang HT, Qin DC, Jin HF, Zhao Y, She MC. MiR-212 exerts suppressive effect on SKOV3 ovarian cancer cells through targeting HBEGF. Tumour Biol. 2014;35:12427–12434. doi: 10.1007/s13277-014-2560-2. [DOI] [PubMed] [Google Scholar]
- 10.Hanlon K, Rudin CE, Harries LW. Investigating the targets of MIR-15a and MIR-16-1 in patients with chronic lymphocytic leukemia (CLL) PLoS One. 2009;4:e7169. doi: 10.1371/journal.pone.0007169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, degli Uberti EC. miR-15a and miR-16-1 down-regulation in pituitary adenomas. J Cell Physiol. 2005;204:280–285. doi: 10.1002/jcp.20282. [DOI] [PubMed] [Google Scholar]
- 12.Di Leva G, Croce CM. The role of microRNAs in the tumorigenesis of ovarian cancer. Front Oncol. 2013;3:153. doi: 10.3389/fonc.2013.00153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Bhattacharya R, Nicoloso M, Arvizo R, Wang E, Cortez A, Rossi S, Calin GA, Mukherjee P. MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res. 2009;69:9090–9095. doi: 10.1158/0008-5472.CAN-09-2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 15.Pietruszewska W, Bojanowska-Poźniak K, Kobos J. Matrix metalloproteinases MMP1, MMP2, MMP9 and their tissue inhibitors TIMP1, TIMP2, TIMP3 in head and neck cancer: An immunohistochemical study. Otolaryngol Pol. 2016;70:32–43. doi: 10.5604/00306657.1202546. [DOI] [PubMed] [Google Scholar]
- 16.Deng J, Wang L, Chen H, Hao J, Ni J, Chang L, Duan W, Graham P, Li Y. Targeting epithelial-mesenchymal transition and cancer stem cells for chemoresistant ovarian cancer. Oncotarget. 2016;7:55771–55788. doi: 10.18632/oncotarget.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou XM, Zhang H, Han X. Role of epithelial to mesenchymal transition proteins in gynecological cancers: Pathological and therapeutic perspectives. Tumour Biol. 2014;35:9523–9530. doi: 10.1007/s13277-014-2537-1. [DOI] [PubMed] [Google Scholar]
- 18.McCubrey JA, Fitzgerald TL, Yang LV, Lertpiriyapong K, Steelman LS, Abrams SL, Montalto G, Cervello M, Neri LM, Cocco L, et al. Roles of GSK-3 and microRNAs on epithelial mesenchymal transition and cancer stem cells. Oncotarget. 2017;8:14221–14250. doi: 10.18632/oncotarget.13991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/β-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 2015;51:1638–1649. doi: 10.1016/j.ejca.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 20.Zhu L, Fang J. The structure and clinical roles of MicroRNA in colorectal cancer. Gastroenterol Res Pract. 2016;2016:1360348. doi: 10.1155/2016/1360348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shukla KK, Misra S, Pareek P, Mishra V, Singhal B, Sharma P. Recent scenario of microRNA as diagnostic and prognostic biomarkers of prostate cancer. Urol Oncol. 2017;35:92–101. doi: 10.1016/j.urolonc.2016.10.019. [DOI] [PubMed] [Google Scholar]
- 22.Shah MY, Ferrajoli A, Sood AK, Lopez-Berestein G, Calin GA. microRNA therapeutics in cancer-an emerging concept. EBioMedicine. 2016;12:34–42. doi: 10.1016/j.ebiom.2016.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pal MK, Jaiswar SP, Dwivedi VN, Tripathi AK, Dwivedi A, Sankhwar P. MicroRNA: A new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biol Med. 2015;12:328–341. doi: 10.7497/j.issn.2095-3941.2015.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X, Ling N, Bai Y, Dong W, Hui GZ, Liu D, Zhao J, Hu J. MiR-16-1 plays a role in reducing migration and invasion of glioma cells. Anat Rec (Hoboken) 2013;296:427–432. doi: 10.1002/ar.22626. [DOI] [PubMed] [Google Scholar]
- 25.Wu WL, Wang WY, Yao WQ, Li GD. Suppressive effects of microRNA-16 on the proliferation, invasion and metastasis of hepatocellular carcinoma cells. Int J Mol Med. 2015;36:1713–1719. doi: 10.3892/ijmm.2015.2379. [DOI] [PubMed] [Google Scholar]
- 26.Ekinci T, Ozbay PO, Yiğit S, Yavuzcan A, Uysal S, Soylu F. The correlation between immunohistochemical expression of MMP-2 and the prognosis of epithelial ovarian cancer. Ginekol Pol. 2014;85:121–130. doi: 10.17772/gp/1702. [DOI] [PubMed] [Google Scholar]
- 27.Tan H, He Q, Gong G, Wang Y, Li J, Wang J, Zhu D, Wu X. miR-382 inhibits migration and invasion by targeting ROR1 through regulating EMT in ovarian cancer. Int J Oncol. 2016;48:181–190. doi: 10.3892/ijo.2015.3241. [DOI] [PubMed] [Google Scholar]
- 28.Amankwah EK, Lin HY, Tyrer JP, Lawrenson K, Dennis J, Chornokur G, Aben KK, Anton-Culver H, Antonenkova N, Bruinsma F, et al. Epithelial-mesenchymal transition (EMT) gene variants and epithelial ovarian cancer (EOC) risk. Genet Epidemiol. 2015;39:689–697. doi: 10.1002/gepi.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takai M, Terai Y, Kawaguchi H, Ashihara K, Fujiwara S, Tanaka T, Tsunetoh S, Tanaka Y, Sasaki H, Kanemura M, et al. The EMT (epithelial-mesenchymal-transition)-related protein expression indicates the metastatic status and prognosis in patients with ovarian cancer. J Ovarian Res. 2014;7:76. doi: 10.1186/1757-2215-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Li X, Zhu Y, Yang P. MicroRNA-16 suppresses epithelial-mesenchymal transition-related gene expression in human glioma. Mol Med Rep. 2014;10:3310–3314. doi: 10.3892/mmr.2014.2583. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Zhang Y, Wu Q, Wang Y-B, Wang W. miR-16 mimics inhibit TGF-β1-induced epithelial-to-mesenchymal transition via activation of autophagy in non-small cell lung carcinoma cells. Oncol Rep. 2018;39:247–254. doi: 10.3892/or.2017.6088. [DOI] [PubMed] [Google Scholar]
- 32.Bodnar L, Stanczak A, Cierniak S, Smoter M, Cichowicz M, Kozlowski W, Szczylik C, Wieczorek M, Lamparska-Przybysz M. Wnt/β-catenin pathway as a potential prognostic and predictive marker in patients with advanced ovarian cancer. J Ovarian Res. 2014;7:16. doi: 10.1186/1757-2215-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arend RC, Londoño-Joshi AI, Straughn JM, Jr., Buchsbaum DJ. The Wnt/β-catenin pathway in ovarian cancer: A review. Gynecol Oncol. 2013;131:772–779. doi: 10.1016/j.ygyno.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Ginther C, Kim J, Mosher N, Chung S, Slamon D, Vadgama JV. Expression of Wnt3 activates Wnt/β-catenin pathway and promotes EMT-like phenotype in trastuzumab-resistant HER2-overexpressing breast cancer cells. Mol Cancer Res. 2012;10:1597–1606. doi: 10.1158/1541-7786.MCR-12-0155-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon YJ, Baek HS, Ye DJ, Shin S, Kim D, Chun YJ. CYP1B1 enhances cell proliferation and metastasis through induction of EMT and activation of Wnt/β-catenin signaling via Sp1 upregulation. PLoS One. 2016;11:e0151598. doi: 10.1371/journal.pone.0151598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonderegger S, Haslinger P, Sabri A, Leisser C, Otten JV, Fiala C, Knöfler M. Wingless (Wnt)-3A induces trophoblast migration and matrix metalloproteinase-2 secretion through canonical Wnt signaling and protein kinase B/AKT activation. Endocrinology. 2010;151:211–220. doi: 10.1210/en.2009-0557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.