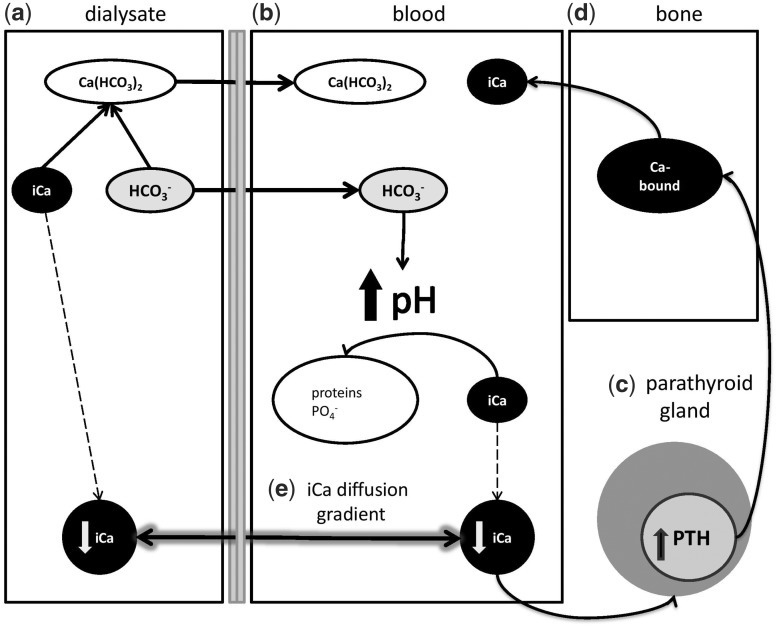
FIGURE 3.
Schematic illustration of intradialytic calcium flux. (a) The reaction of bicarbonate and iCa in dialysate leads to the formation of Ca(HCO3)2 and reduces dialysate iCa concentration. Ca(HCO3)2 diffuses probably through the membrane after the concentration gradient to the blood. (b) The bicarbonate load from dialysate alkalinized plasma to form water and CO2 (risk of hypercapnia). An increase of plasma pH facilitates iCa binding to plasma anions (protein, phosphate) and results in a decrease of plasma iCa (biologically active form of Ca) (c). This increases PTH secretion, which stimulates the release of iCa from bone (d). (e) The direction of iCa shift is determined by iCa gradient between dialysate and plasma, which depends on the degree of intradialytic plasma alkalization. The greater difference of plasma and dialysate pH leads to the greater plasma iCa decrease and greater iCa gain during dialysis.