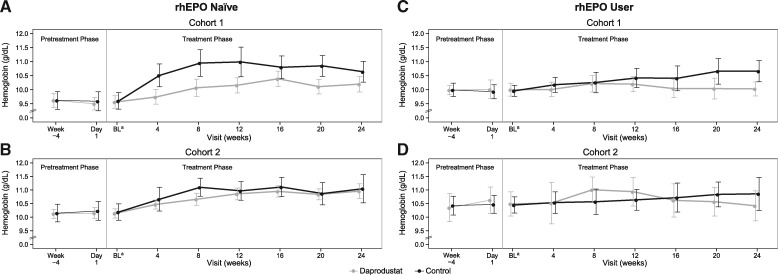
FIGURE 2:
Mean and 95% confidence interval for hemoglobin over time by original (A) and amended (B) hemoglobin criteria for the rhEPO-naïve group and original (C) and amended (D) hemoglobin criteria for the rhEPO user group. For Cohort 1, the hemoglobin entry criterion was 8–10 g/dL in the rhEPO-naïve group and 9–10.5 g/dL in rhEPO users, and the hemoglobin target range was 9–10.5 g/dL. For Cohort 2, the hemoglobin entry criterion was 8–11 g/dL in the rhEPO-naïve group and 9–11.5 g/dL in rhEPO users, and the hemoglobin target range was 10–11.5 g/dL. aBaseline values were calculated as the average of measurements at Week-4 and Day 1. BL, baseline; rhEPO, recombinant human erythropoietin.