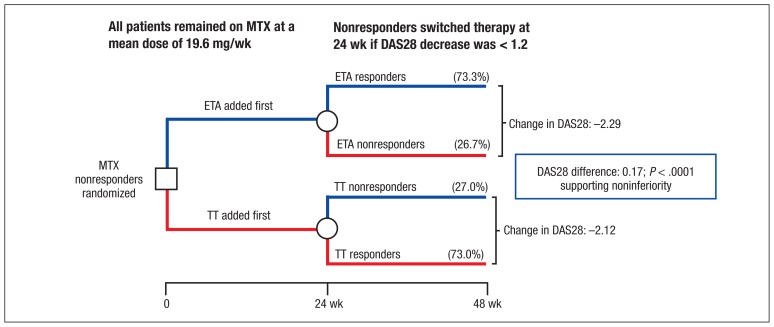
Figure 1.
The Study Design and Primary Endpoint of the 48-Week RACAT Trial
Double-blind strategy that randomized MTX nonresponders to either etanercept plus MTX or sulfasalazine and hydroxychloroquine plus MTX (triple therapy). Disease activity was assessed at 24 weeks, and if the response was not adequate (DAS28 of 1.2 or more), the patient was switched to the other therapy. The change of DAS28 at 48 weeks was the primary outcome. Reprinted with permission of the Massachusetts Medical Society, copyright 2013.4
Abbreviations: DAS28, disease activity score; ETA, etanercept therapy, MTX, methotrexate; RACAT, Rheumatoid Arthritis: Comparison of Active Therapies; TT, triple therapy.