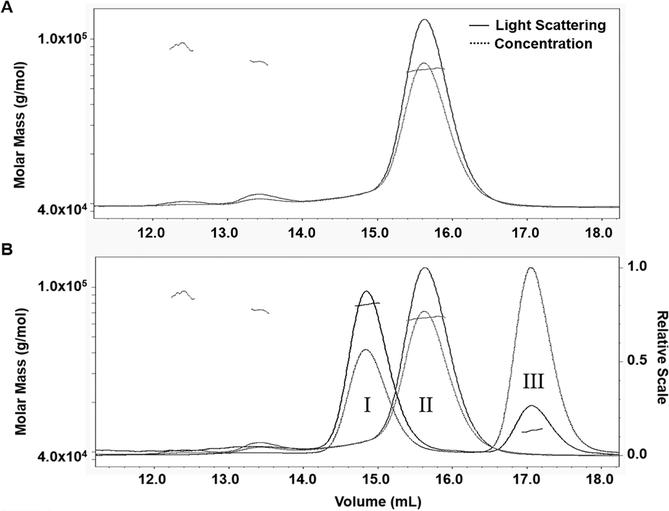
Figure 2:
Determination of the heterodimeric nature of the soluble Cry1Ab-EC12 complex and its individual monomeric components by SEC-MALS analysis. (A) SEC-MALS analysis for purified Cry1Ab toxin. Elution volume is indicated in ml and molar mass is measured in g/mol. The solid-line curve represents light scattering and the dotted-line curve indicates abundance of the proteins. The slanted dotted lines signify homogeneity of the sample. (B) SEC-MALS analysis for the Cry1Ab-EC12 mixture. Results were merged with the SEC-MALS analysis of Cry1Ab toxin. The SEC-MALS analysis of the Cry1Ab-EC12 mixture produced peaks I and III and the Cry1Ab alone was present in peak II, which is the single major peak in Panel A. The merged results show relative elution volumes of all three proteins, and the relative scale of each protein peak is indicated by the Y-axis on the right side. Horizontal dotted lines for all three proteins indicate their homogeneity.