Abstract
Introduction:
Despite decades of focused research efforts, cancer remains a significant cause of morbidity and mortality. Tumor necrosis factor(TNF)-related apoptosis-inducing ligand (TRAIL) is capable of inducing cell death selectively in cancer cells while sparing normal cells.
Areas covered:
In this review, the authors cover TRA therapy and strategies that have been undertaken to improve their efficacy, as well as unconventional approaches to TRAIL pathway activation including TRAIL-inducing small molecules. They also discuss mechanisms of resistance to TRAIL and the use of combination strategies to overcome it.
Expert commentary:
Targeting the TRAIL pathway has been of interest in oncology, and although initial clinical trials of TRAIL receptor agonists (TRAs) showed limitations, novel approaches represent the future of TRAIL-based therapy.
Keywords: Apoptosis, cancer, TRAIL, DISC, apoptosome, TRAIL-R agonist, Imipridone, atrimer
1. Introduction
Apoptosis, activated under pathological and physiological conditions, results in the programmed destruction of a cell. The hallmarks of apoptosis include cell shrinkage, nuclear DNA fragmentation, and membrane blebbing [1]. Apoptosis can be triggered following the activation of the cell-intrinsic pathway or the cell extrinsic pathway. Activation of the intrinsic, or mitochondrial, pathway is regulated by the B-cell lymphoma-2 (Bcl-2) family of proteins. The Bcl-2 family includes pro-apoptotic proteins and anti-apoptotic proteins, all of which function to control the permeability of the mitochondrial outer membrane [2]. Cellular stresses such as DNA damage activate pro-apoptotic and inhibit anti-apoptotic Bcl-2 family proteins in a p53-dependent manner. This results in mitochondrial outer membrane permeabilization (MOMP) and release of cytochrome c. Cytochrome c associates with Apaf-1 and activator caspase-9, forming the apoptosome [3]. Caspase-9, as a part of the apoptosome, can cleave and activate effector caspase-3 [4], which goes on to cleave downstream targets and induce cell death.
The extrinsic pathway is activated following binding of pro-apoptotic stimuli such as tumor necrosis factor(TNF)-related apoptosis-inducing ligand (TRAIL) to cell surface receptors. TRAIL/Apo2L was discovered as an inducer of apoptosis [5, 6] that, unlike other TNF superfamily members, selectively targets cancer cells while leaving normal cells unharmed [7]. TRAIL binds to cell surface receptors DR4 (TRAIL-R1) [8] and DR5 (TRAIL-R2) [9] as a homotrimer [10]. DR4 and DR5 have functional intracellular carboxyl terminal death domains (DDs), and ligand binding leads to receptor oligomerization and recruitment of adaptor protein Fas-associated death domain (FADD). Alternative TRAIL receptors TRAIL-R3, TRAIL-R4, and the soluble osteoprotegerin (OPG) lack a viable DD [11–13]. TRAIL-R3 and TRAIL-R4 may function to act as decoy receptors and inhibit TRAIL-induced apoptosis [14]. Through its death effector domain (DED), FADD recruits pro-caspase-8 and pro-caspase-10 [15, 16]. Proper formation of this death inducing signaling complex (DISC) is required for apoptosis induction. Once recruited to the DISC, pro-caspase-8 and −10 are cleaved and activated [17]. In type I cells, caspase-8 cleavage is sufficient to induce cleavage of caspase-3 and cell death. In type II cells, an extra step is required: caspase-8 mediated BH3-interacting-domain death agonist (BID) cleavage activates the intrinsic apoptosis pathway [18]. The cleaved form of Bcl-2 family protein BID, tBID, enables mitochondrial outer membrane permeabilization (MOMP) and cytochrome c release [19, 20]. Release of mitochondrial cytochrome c is necessary for formation of the apoptosome and ultimately caspase-3 cleavage and the induction of cell death in type II cells.
TRAIL is expressed on the surface of activated NK cells [21] and can bind to death receptors expressed on tumor cells, triggering their apoptosis [22, 23]. TRAIL-deficient mice are more susceptible to tumor initiation and metastasis following carcinogen exposure, in a partially NK-cell-dependent manner [24]. Similarly, TRAIL-deficient mice show increased susceptibility to the growth of primary tumors and their spontaneous liver metastasis following inoculation of syngeneic breast cancer cells [25]. In addition to triggering apoptosis, death receptor engagement can activate pro-inflammatory transcription factor NF-KB [26–28] and induce TRAIL-resistant cancer cells to proliferate [29] and secrete tumor promoting cytokines [30, 31].The mechanism by which TRAIL activates NF-KB involves nuclear translocation following phosphorylation and degradation of inhibitor of KB (IKB) proteins [32].
The identification of TRAIL as an extrinsic apoptosis pathway ligand with such a strong selectivity for cancer cells over normal cells [7] sparked an interest in developing TRAIL receptor agonists (TRAs) as anti-cancer therapeutics.
2. TRAIL-R agonists
2.1: Soluble recombinant human TRAIL
Two subtypes of TRAs have been developed for use in the clinic: recombinant forms of the TRAIL protein and DR4/5 agonistic antibodies. The initial efficacy of recombinant human TRAIL against cancer cells was shown in the early 1990’s [10]. Early forms of recombinant TRAIL contained poly-histidine or FLAG epitope tags [5, 6] used to aid in the protein purification process. These forms of TRAIL showed toxicity against primary hepatocytes in vitro [33], which prompted concern about potential in vivo hepatotoxicity until it was determined that untagged versions of the molecule did not have the same effect [34]. Recombinant TRAIL was clinically developed as rhApo2L.0/dulanermin. Preclinically, dulanermin binds to both DR4 and DR5 to induce cell death in cancer cells but not normal cells [10]. While safe and well tolerated in patients [35], the protein was also disappointingly inactive when tested in the setting of a randomized phase II trial [36]. The protein’s extremely short half-life [37] has been blamed for these discouraging results. The second class of TRAs is made up of monoclonal death receptor binding antibodies. These antibodies boasted a half-life on the order of days, much longer than that of dulanermin, the plasma concentration of which halves in under an hour after injection.
2.2: TRAIL receptor agonistic antibodies
Death receptor agonistic antibodies that have been tested clinically include one DR4 agonistic antibody (mapatumumab), and five DR5 agonistic antibodies (drozitumumab, conatumumab, lexatumumab, tigatuzumab, LBY-135). A recent study compared the efficacy of anti-DR5 antibody tigatuzumab plus nanoparticle albumin-bound paclitaxel or nanoparticle albumin-bound paclitaxel alone in patients with triple negative breast cancer. Prolonged survival of several patients treated with the combination support further study of these agents [38]. While TRA antibodies including tigatuzumab have been generally well tolerated, they have not yet advanced into phase III.
3. Strategies for improving the efficacy of TRAIL-R agonists
3.1: Increasing the stability and trimerization ability of TRAIL
Despite the unsatisfactory performance of early TRAs in the clinic, scientists and clinicians are not ready to give up on targeting the TRAIL pathway. Cell death induction by TRAIL requires ligand trimerization prior to death receptor binding. Tagged formats of recombinant human TRAIL with trimerization domains were initially abandoned for development after the discovery that his-TRAIL and FLAG-TRAIL exhibited toxicity against primary hepatocytes [33]. TRAIL with an attached leucine zipper motif (LZ-TRAIL) [7], an attached isoleucine zipper motif (iz-TRAIL) [39], and an attached tenascin-C oligomerization domain (TNC-TRAIL) [40] effectively trimerize and are non-toxic to hepatocytes [39]. These forms of TRAIL have been shown to be more potent and have longer half-lives than rhApo2L.0/dulanermin, the untagged version of TRAIL used in clinical trials. Permutation of the TRAIL amino acid sequence has been undertaken to improve pharmacokinetic properties and increase potency of killing. The single chain TRAIL trimer (scTRAIL) protein is expressed as three linker-connected TRAIL extracellular domains [41], eliminating the need for trimerization in vivo. Recombinant mutant human TRAIL (rmhTRAIL), also known as circularly permutated TRAIL (CPT) contains a flexible linker connecting the N terminus of amino acids 121–135 to the C terminus of amino acids 135–281 [42]. Both novel TRAIL forms show increased potency above the wildtype protein [41, 42]. CPT has been tested in a phase II study of patients with relapsed and refractory multiple myeloma in combination with thalidomide. The protein was shown to be well-tolerated and have an overall response rate (ORR) of 22% [43]. An alternative approach to improve the efficacy of TRAIL by increasing its stability has involved the covalent linkage of TRAIL to polyethylene glycol (PEGylated TRAIL) [44].
3.2: Directly targeting TRAIL to cancer cells
In addition to making alterations to the TRAIL protein itself, researchers have devised creative delivery strategies to effectively target TRAIL to cancer cells. Anticancer therapeutics can be coupled with components such as lipids or polymers to form nanoparticles. Nanoparticle delivery to tumor tissue relies on a phenomenon called the enhanced permeability and retention (EPR) effect. Solid tumors possess abnormal blood and lymphatic vessels with increased permeability compared to those found in healthy tissues, and as a result, macromolecules will leak out of vessels and collect within the tumor [45]. A variety of TRAIL-containing nanoparticles are under development. Some groups have attached and immobilized TRAIL onto the surface of the nanoparticle and other have encapsulated TRAIL within. TRAIL-containing nanoparticles have been reviewed in greater detail by de Miguel et. al [46]. Fusion proteins combining the antigen specific single-chain variable-region (scFv) of an antibody with the TRAIL protein represent an alternative strategy for targeting TRAIL to cancer cells. scFv regions targeting a variety of antigens expressed on cancer cells and immune cells have been developed. TRAIL-scFv fusion proteins have been reviewed in greater detail by de Bruyn et. al [47].
4. Alternative approaches to TRAIL pathway activation
4.1: TRAIL-inducing (imipridone) compounds
A novel approach for targeting the TRAIL pathway involves small molecules and peptide TRAIL pathway activators. ONC201, an anti-cancer compound belonging to the novel imipridones class, was originally identified in a luciferase reporter screen as a p53-independent transcriptional inducer of the TRAIL gene [48]. ONC201 was selected as the lead candidate from the screen following its success in preclinical studies, where it exhibited a favorable safety profile and showed anti-tumor effects in vivo with a single dose [49]. The mechanism by which ONC201 induces transcription of the TRAIL gene involves inactivation of pro-survival kinases Akt and ERK, leading to decreased phosphorylation of transcription factor FOXO3a. Dephosphorylated FOXO3a is activated and translocates to the nucleus to activate its target gene TRAIL [49]. More recently, it was determined that ONC201 also induces cell death through activation of a PERK-independent ISR [50, 51]. The compound also exerts TRAIL dependent [52] anti-cancer stem cell activity [52, 53]. ONC201 has shown efficacy in against a variety of tumor types preclinically including breast cancer [54], glioblastoma [55], pancreatic cancer, and prostate cancer. The compound has also been shown the recruit NK cells to tumors [56]. The compound has completed its first-in-human clinical trial in advanced solid tumors [57] and is currently being tested in multiple phase II trials. Analogs of ONC201 able to upregulate TRAIL with increased potency have been synthesized [58] and will hopefully enter the clinic soon.
4.2: TRAIL-R activating atrimers, TRAIL-R activating small molecules
Other unconventional approaches to activating the TRAIL pathway have included small DR5 binding peptides [59], DR5 activating small molecules that induce receptor clustering and aggregation [60], and trivalent DR4 atrimer complexes [61].
5. TRAIL resistance and the use of combination therapy to overcome it
5.1: TRAIL resistance is common in tumor cells
While the use of TRAs with increased potency and ability to target to cancer cells is a valid approach, it does not address the problem of TRAIL resistance. Resistance mechanisms have been identified at multiple points in the TRAIL pathway, from cell surface ligand binding to intracellular caspase cleavage. The pathway became of interest as a therapeutic target due to its ability to induce cell death in cancer cell while sparing normal cells [7]. Different mechanisms of resistance to TRAIL have been identified in normal cells, including post-translational modification of caspase-8 [62], high levels of c-FLIP [63, 64] and cell surface decoy receptors [64].
5.2: Resistance at the level of the death receptors
In cancer cells, low surface expression of TRAIL receptors DR4 and DR5 correlates with decreased sensitivity to TRAIL. Specific genetic and epigenetic changes responsible for decreased DR4 and DR5 expression have been identified and include promoter hypermethylation [65, 66], loss-of-function mutations [67, 68], gene deletion [69]. Impaired death receptor transport to [70] and constitutive receptor endocytosis from the cell surface [71] also modulate cell surface expression and thus TRAIL sensitivity. Therapies that induce DNA damage and activate p53, such as chemotherapy and radiation, can upregulate DR4 [72] and DR5 [73] and have been tested in combination with TRAIL. Strategies to overcome TRAIL resistance associated with low cell surface death receptor levels have also included combinations with histone deacetylase (HDAC) inhibitors [74, 75], proteasome inhibitors [76], and ER stress inducer tunicamycin [77]. Interestingly, post-translational modifications of death receptors can also modulate TRAIL sensitivity. O-glycosylation of DR4 and DR5 enhances ligand-mediated receptor clustering, DISC formation, and caspase activation. Genes encoding o-glycosylating enzymes are overexpressed in TRAIL-sensitive cells, and their inhibition causes resistance [78].
5.3: Resistance at the level of the caspase-8 and c-FLIP
Resistance can also occur at the level of caspase-8. Deletion and hypermethlyation of the caspase-8 gene has been observed in childhood neuroblastomas [79] and small cell lung carcinomas [66]. An endogenous mediator of TRAIL resistance is cellular flice inhibitory protein (c-FLIP). Like caspase-8, FLIP contains a death-effector domain (DED) that allows it to bind to FADD. Unlike caspase-8, c-FLIP lacks the protease activity that is required for proper cleavage of effector caspases and thus apoptosis induction [80]. The short splice variant of c-FLIP, c-FLIPs, prevents caspase-8 cleavage at the DISC [81]. High c-FLIP expression is associated with resistance to TRAIL in cancer cells, as reviewed by Newsom-Davis [82]. Interestingly, c-myc has been shown to modulate TRAIL sensitivity through negative regulation of c-FLIP transcription [83]. Reduction of c-FLIP expression by a variety of compounds, including HDAC inhibitor LBH589 [84] and multiple PPARγ ligands [85], sensitizes to TRAIL-mediated apoptosis.
5.4: Resistance mediated by B-cell lymphoma-2 (Bcl-2) family proteins
Pro-apoptotic Bcl-2 family protein Bid is highly relevant to TRAIL-induced apoptosis, as discussed previously. In type II cells, caspase-8 mediated Bid cleavage and activation is required for induction of the intrinsic apoptosis pathway and cell death [18]. Type II cancer cells can develop resistance to TRAIL following overexpression of anti-apoptotic Bcl-2 family proteins Mcl-1 [86] and Bcl-2 [87] or loss of pro-apoptotic Bcl-2 family protein Bax [88]. BH3-mimetics designed to antagonize anti-apoptotic proteins are being tested clinically and can sensitize cancer cells to TRAIL [89, 90].
5.5: Resistance mediated by Inhibitor of apoptosis (IAP) family proteins
A class of caspase inhibitors known as the inhibitor of apoptosis (IAP) family proteins can also regulate TRAIL-mediated cell death. IAP proteins such as XIAP contain baculovirus IAP repeat domains, essential for direct binding to and inhibition of caspases 3,7, and 9 [91]. Smac/DIABLO, an endogenous XIAP inhibitor, is released following MOMP, binds to XIAP, and inhibits its function [92, 93]. Small molecule Smac mimetics with the capability to antagonize IAPs are being tested in the clinic. These compounds sensitize cancer cells to TRAIL-mediated apoptosis in preclinical models [94, 95]. Other IAP proteins, including cellular-IAP1 (c-IAP1) and cellular-IAP2 (c-IAP2) are able to ubiquitinate protein targets, including caspase-3 and caspase-7, and target them for degradation [96] through an E3-ligase Really Interesting New Gene (RING) domain [97]. Interestingly, multikinase inhibitor sorafenib has been shown to sensitize resistant cells to TRAIL through downregulation of Mcl-1, c-IAP2, and c-FLIP [98, 99] through inhibition of the JAK/STAT3 signaling pathway [100].
5.6: Resistance mediated by PI3K-Akt signaling
Phosphatidylinositol 3-phosphate kinase (PI3K) is a well-studied regulator of cellular proliferation, growth, and survival. Paradoxically, treatment with TRAIL can activate this prosurvival pathway [101]. Binding of growth factors to cell surface PI3K receptors leads to the generation of second messenger phosphotidylinositol-3,4,5-triphosphate (PIP3). PIP3 binds to kinase Akt, which regulates many downstream targets such as mammalian target of rapamycin (mTOR) complex 1 and 2 through phosphorylation. Activating mutations of PIK3CA, the gene that encodes the p110alpha catalytic subunit of PI3K, may confer resistance to TRAIL [102]. Similary, loss of expression of PI3K negative regulator PTEN or overexpression of its downstream target Akt lead to decreased TRAIL sensitivity [103]. Combination of TRAIL with PI3K [104], Akt [105], and mTORC1 inhibitors [106] enhances apoptosis.
6. Conclusion
Interest in the TRAIL pathway developed following the observation that TRAIL could induce cell death in transformed cells but showed no toxicity towards normal cells [7]. TRAIL receptor agonists have been developed for use in the clinic and include recombinant human soluble TRAIL and death receptor agonistic antibodies. Unfortunately, these approaches have yet to show efficacy in a clinical trial. Multiple strategies have been used to improve the efficacy of these agonists. Forms of TRAIL with increased stability have been developed, and multiple systems for improved TRAIL delivery have been identified. A novel approach for TRAIL pathway activation involves small molecule and peptide TRAIL pathway activators. ONC201, originally called TRAIL inducing compound 10 (TIC10) is one such small molecule that is currently being tested in clinical trials. TRAIL resistance in cancer cells is a significant problem, and combination therapies with existing drugs have been explored as a method for combating it. In conclusion, although early TRA therapies showed limited clinical efficacy, novel and innovative approaches have potential and merit further clinical testing.
7. Expert Commentary
The TRAIL pathway holds enormous potential among the therapeutic strategies currently employed to treat cancer. The field has evolved significantly since the 1980’s when significant toxicities with TNF were being observed in clinical trials. There have been hurdles in the development of TRAIL as a therapeutic and the field has shifted towards TRAIL receptor agonists or other TRAIL pathway activating small molecules. Activation of the TRAIL pathway which is part of the innate immune system for cancer and metastasis suppression offers hope and a bright future given that tumors with various oncogenic drivers can be targeted. Unlike TNF, the TRAIL ligand as well as TRAIL receptor agonists have generally proven to not be limited by toxicity in the clinic. There was some concern early on that hepatic expression of DR5 might result in more toxicity from therapeutic targeting of TRAIL-R2 versus TRAIL-R1 in the clinic. However, this did not materialize in the clinical trials. Of course, neither ant-DR5 or anti-DR4 alone is equivalent to TRAIL and it is complicated to combine 2 unapproved therapeutics in early phase trials. Thus, the answers are unfortunately not there at present to address the issue of whether targeting TRAIL-R1 (DR4) or TRAIL-R2 (DR5) or both in the path forward in the clinic.
The TRAIL pathway is active in cancer suppression despite p53 tumor suppressor gene mutations or common oncogene mutations such as KRAS, BRAF, EGFR, among others. While there are numerous TRAIL pathway resistance mechanisms in cancer including TRAIL decoys, TRAIL receptor glycosylation and translocation to the cell membrane, NFkB, Bcl-XL, Mcl-1, the IAP family, Akt, FLIP, mutation or loss of caspase 8 expression, there are also promising combinatorial therapeutics that can address resistance. There are effects of TRAIL on the tumor microenvironment that need to be further examined in treated human tumors in the clinic with regard to a potential immune suppressive milieu. The development of predictive biomarkers for specific TRAIL pathway therapeutics or combinations would help advance the field and translation to the clinic. Unfortunately, no reliable predictive biomarkers have emerged in the clinical trials to help with our understanding of response or resistance of specific patients to TRIAL receptor targeted therapeutics, despite available preclinical data. This includes M3, glycosylated receptors, TRAIL-R expression, or a variety of intracellular biomarkers. There is opportunity for a more integrated genomic and proteomic approach to address this unmet need in the future.
It remains to be seen in the clinic what resistance mechanisms ultimately will prove to be insurmountable by TRAIL pathway-directed monotherapy and which mechanisms may be overcome by combination therapy including immunotherapy. The major limitation to combination therapeutics revolves around which TRAIL receptor or TRAIL pathway targeting therapeutic will ultimately serve as the anchor for a combination regimen. It remains for future development to establish specific therapy combinations targeting specific tumor types. Certainly, the combination of a TRAIL-R2 agonist antibody plus docetaxel for triple negative breast cancer holds promise. The combination of TRAIL or TRAIL receptor agonistic targeting plus sorafenib has much support mostly from preclinical data. While anti-DR4 plus sorafenib was disappointing in hepatocellular cancer, the reason for failure has remained unclear, and it is possible that with better patient selection such a combination could be more effective. A number of other combinations are emerging from preclinical studies, e.g. with ONC201 such as combination with anti-VEGF targeting, anti-PD-1 targeting, sorafenib, or everolimus, among others. Clinical trials with specific combinations in specific indications will ultimately direct the most promising agent combinations towards further development. The TRAIL pathway remains a powerful host mechanism for cancer suppression and one that has not yet been adequately exploited for patient benefit in oncology.
8. Five-year view
There are currently no FDA-approved agents specifically targeting activation of the TRAIL pathway. The TRAIL pathway is a powerful innate immune tumor suppressive mechanism that has yet to be harnessed in cancer therapy. The availability of TRAIL receptor agonist antibodies or TRAIL pathway stimulating/activating small molecules such as ONC201 in multiple clinical trials points towards the future. It is expected that while the agents have single agent anti-tumor effects, the combinations for specific tumor types is likely the path forward. Moreover, in the era of precision medicine and careful patient selection, there is expectation that the patients most likely to respond would be the ones who are selected for specific therapy combinations. In the case of ONC201, preliminary indications for H3K27M mutated gliomas such as DIPG, with altered dopamine receptor DRD2 expression appear promising. How the dopamine receptor expression plays out in predicting efficacy of ONC201 or analogues for other tumor types is expected to be unraveled from ongoing or yet to be initiated clinical trials.
Figure 1: Signaling pathways linking the TRAIL ligand to apoptosis.
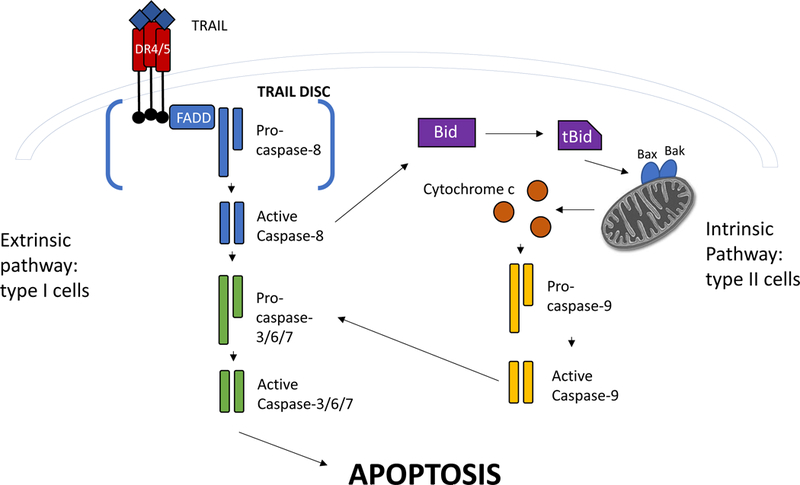
TRAIL binds to its cell surface receptors DR4 and DR5 as a trimer. Receptor clustering and recruitment of FADD to intracellular the death domains starts formation of the DISC. Association of pro-caspase-8 finalizes DISC formation. Caspase-8 is cleaved to its active form. In type I cells, caspase-8 cleavage will directly cleave effector caspases 3, 6, and 7, triggering apoptosis. In type II cells, a secondary signal through the mitochondria is required. Caspase-8 will cleave Bcl-2 family protein Bid to its truncated form, tBid. This enables permeabilization of the mitochondrial outer membrane and release of cytochrome c into the cytosol. Pro-caspase-9 will be activated and go on to cleave effector caspases 3,6, and 7, leading to the induction of apoptosis.
Table 1:
Summary of the therapeutic approaches used to target the TRAIL pathway.
| Therapeutic Approach | Stage in Development |
|---|---|
| TRAIL-receptor agonists | |
| his-TRAIL/FLAG TRAIL | preclinical |
| rhApo2L.0/dulanermin | discontinued |
| DR4 agonistic monoclonal antibodies | |
| mapatumumab | Phase II |
| DR5 agonistic monoclonal antibodies | |
| drozitumab | discontinued |
| conatumumab | Phase II |
| lexatumumab | discontinued |
| tigatumumab | Phase II |
| LBY-135 | discontinued |
|
Strategies for improving the
efficacy of TRAIL-receptor agonists |
|
| LZ-TRAIL | preclinical |
| iz-TRAIL | preclinical |
| TNC-TRAIL | preclinical |
| scTRAIL | preclinical |
| CPT | discontinued |
| PEGylated TRAIL | preclinical |
| TRAIL containing nanoparticles | preclinical |
| TRAIL/scFv fusion proteins | preclinical |
| TRAIL expressing mesenchymal stem cells | Phase I/II |
| TRAIL pathway activation | |
| TRAIL-inducing (imipridone) compounds | Phase I/II |
| TRAIL-R activating atrimers | preclinical |
| TRAIL-R activating small molecules/peptides | preclinical |
Key issues.
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is capable of inducing cell death selectively in cancer cells while sparing normal cells. Targeting the TRAIL pathway has been of interest in oncology since the 1990’s.
Limited clinical efficacy has been observed in trials of recombinant human TRAIL (rhTRAIL) or death receptor agonistic antibodies.
Novel approaches for targeting the TRAIL pathway involve directly upregulating TRAIL and its receptors or using combination therapies to target resistance mechanisms.
Acknowledgments
Funding
This article was funded by NIH grant R01 CA173453.
Footnotes
Declaration of Interest
WS El-Deiry is a co-Founder and shareholder in Oncoceutics, a company that is developing ONC201 in the clinic. WS El-Deiry is compliant with institutional and NIH disclosure guidelines. Oncoceutics provided no financial support for the review and has not been involved in any way in its writing. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Hengartner MO, The biochemistry of apoptosis. Nature, 2000. 12(407): p. 770–776. [DOI] [PubMed] [Google Scholar]
- 2.Youle RJ and Strasser A, The BCL-2 protein family: opposing activities that mediate cell death. Nature Reviews Molecular Cell Biology, 2008. 9. [DOI] [PubMed] [Google Scholar]
- 3.Cain K, Bratton SB, Langlais C, et al. , Apaf-1 Oligomerizes into Biologically Active 700-kDa and Inactive 1.4-MDa Apoptosome Complexes. The Journal of Biological Chemistry, 2000. 275(9): p. 6067–6070. [DOI] [PubMed] [Google Scholar]
- 4.Bratton SB, Walker G, Srinivasula SM, et al. , Recruitment, activation and retention of caspases-9 and −3 by Apaf-1 apoptosome and associated XIAP complexes. The EMBO Journal, 2001. 20(5): p. 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pitti RM, Marsters SA, Ruppert S, et al. , Induction of Apoptosis by Apo-2 Ligand, a New Member of the Tumor Necrosis Factor Cytokine Family. The Journal of Biological Chemistry, 1996. 271(22): p. 12687–12690. [DOI] [PubMed] [Google Scholar]
- 6.Wiley SR, Schooley l.K., Smolak l.P.J., et al. , Identification and Characterization of a New Member of the TNF Family that Induces Apoptosis. Immunity, 1995. 3: p. 673–682. **Of considerable importance due to the discovery of an endogenous ligand, and member of the TNF family with therapeutic potential for cancer. [DOI] [PubMed] [Google Scholar]
- 7.Walczak H, Miler RE, Ariail K, et al. , Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nature Medicine, 1999. 5(2): p. 157–163. **Of considerable importance due to the demonstration of preclinical anti-tumor efficacy of TRAIL. [DOI] [PubMed] [Google Scholar]
- 8.Pan G, O’Rourke K, Chinnaiyan AM, et al. , The Receptor for the Cytotoxic Ligand TRAIL. Science, 1997. 276(5309): p. 111–113. [DOI] [PubMed] [Google Scholar]
- 9.Sheridan JP, Marsters SA, Pitti RM, et al. , Control of TRAIL-Induced Apoptosis by a Family of Signaling and Decoy Receptors. Science, 1997. 277(5327): p. 818–821. **Of considerable importance as one of the original papers to identify TRAIL-binding receptors that mediate pro-apoptotic effects and decoys that can protect normal cells. [DOI] [PubMed] [Google Scholar]
- 10.Ashkenazi A, Pai RC, Fong S, et al. , Safety and antitumor activity of recombinant soluble Apo2 ligand. The Journal of Clinical Investigation, 1999. 104(2): p. 155–162. *Of importance as an excellent source for activity of TRAIL in the clinic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Degli-Esposti MA, Dougall WC, Smolak PJ, et al. , The Novel Receptor TRAIL-R4 Induces NF- B and Protects against TRAIL-Mediated Apoptosis, yet Retains an Incomplete Death Domain. Immunity, 1997. 7: p. 813–820. [DOI] [PubMed] [Google Scholar]
- 12.Degli-Esposti MA, Smolak PJ, Walczak H, et al. , Cloning and Characterization of TRAIL-R3, a Novel Member of the Emerging TRAIL Receptor Family. The Journal of Experimental Medicine, 1997. 186(7): p. 1165–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emery JG, McDonnell P, Burke MB, et al. , Osteoprotegerin Is a Receptor for the Cytotoxic Ligand TRAIL. The Journal of Biological Chemistry, 1998. 273(23): p. 14363–14367. [DOI] [PubMed] [Google Scholar]
- 14.Merino D, Lalaoui N, Morizot A, et al. , Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol, 2006. 26(19): p. 7046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kischkel FC, Lawrence DA, Chuntharapai A, et al. , Apo2L/TRAIL-Dependent Recruitment of Endogenous FADD and Caspase-8 to Death Receptors 4 and 5. Immunity, 2000. 12: p. 611–620. [DOI] [PubMed] [Google Scholar]
- 16.Kischkel FC, Lawrence DA, Tinel A, et al. , Death receptor recruitment of endogenous caspase-10 and apoptosis initiation in the absence of caspase-8. J Biol Chem, 2001. 276(49): p. 46639–46. [DOI] [PubMed] [Google Scholar]
- 17.Chen M and Wang J, Initiator caspases in apoptosis signaling pathways. Apoptosis, 2002. 7: p. 313–319. [DOI] [PubMed] [Google Scholar]
- 18.Ozoren N and El-Deiry WS, Defining characteristics of Types I and II apoptotic cells in response to TRAIL. Neoplasia, 2002. 4(6): p. 551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei MC and Korsmeyer SJ, tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes and Development, 2000. 14: p. 2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 20.Luo X, Budihardjo I, Zou H, et al. , Bid, a Bcl2 Interacting Protein, Mediates Cytochrome c Release from Mitochondria in Response to Activation of Cell Surface Death Receptors. Cell, 1998. 94: p. 481–490. [DOI] [PubMed] [Google Scholar]
- 21.Wallin RP, Screpanti V, Michaelsson J, et al. , Regulation of perforin-independent NK cell-mediated cytotoxicity. Eur J Immunol, 2003. 33(10): p. 2727–35. [DOI] [PubMed] [Google Scholar]
- 22.Smyth MJ, Cretney E, Takeda K, et al. , Tumor Necrosis Factor–related Apoptosis-inducing Ligand (TRAIL) Contributes to Interferon-dependent Natural Killer Cell Protection from Tumor Metastasis. J. Exp. Med, 2001. 193(6): p. 661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takeda K, Smyth MJ, Cretney E, et al. , Involvement of tumor necrosis factor-related apoptosis-inducing ligand in NK cell-mediated and IFN-gamma-dependent suppression of subcutaneous tumor growth. Cell Immunol, 2001. 214(2): p. 194–200. [DOI] [PubMed] [Google Scholar]
- 24.Takeda K, Smyth MJ, Cretney E, et al. , Critical Role for Tumor Necrosis Factor-related Apoptosis Inducing Ligand in Immune Surveillance Against Tumor Development. J. Exp. Med, 2002. 195(2): p. 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cretney E, Takeda K, Yagita H, et al. , Increased Susceptibility to Tumor Initiation and Metastasis in TNF-Related Apoptosis-Inducing Ligand-Deficient Mice. The Journal of Immunology, 2002. 168(3): p. 1356–1361. [DOI] [PubMed] [Google Scholar]
- 26.Schneider P, Thome M, Burns K, et al. , TRAIL Receptors 1 (DR4) and 2 (DR5) Signal FADD-Dependent Apoptosis and Activate NF- B. Immunity, 1997. 7: p. 831–836. [DOI] [PubMed] [Google Scholar]
- 27.Berg D, Stuhmer T, Siegmund D, et al. , Oligomerized tumor necrosis factor-related apoptosis inducing ligand strongly induces cell death in myeloma cells, but also activates proinflammatory signaling pathways. FEBS J, 2009. 276(23): p. 6912–27. [DOI] [PubMed] [Google Scholar]
- 28.Jin Z and El-Deiry WS, Distinct signaling pathways in TRAIL- versus tumor necrosis factor-induced apoptosis. Mol Cell Biol, 2006. 26(21): p. 8136–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ehrhardt H, Fulda S, Schmid I, et al. , TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-κB. Oncogene, 2003. 22: p. 3842–3852. [DOI] [PubMed] [Google Scholar]
- 30.Henry CM and Martin SJ, Caspase-8 Acts in a Non-enzymatic Role as a Scaffold for Assembly of a Pro-inflammatory “FADDosome” Complex upon TRAIL Stimulation. Mol Cell, 2017. 65(4): p. 715–729 e5. [DOI] [PubMed] [Google Scholar]
- 31.Hartwig T, Montinaro A, von Karstedt S, et al. , The TRAIL-Induced Cancer Secretome Promotes a Tumor-Supportive Immune Microenvironment via CCR2. Mol Cell, 2017. 65(4): p. 730–742 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y, Devin A, Cook A, et al. , The Death Domain Kinase RIP Is Essential for TRAIL (Apo2L)-Induced Activation of I B Kinase and c-Jun N-Terminal Kinase. Molecular and Cellular Biology, 2000. 20(18): p. 6638–6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Minji J, KIM T-H, SEOL D-W, et al. , Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nature Medicine, 2000. 6(5). [DOI] [PubMed] [Google Scholar]
- 34.Lawrence D, SHAHROKH Z, MARSTERS S, et al. , Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nature Medicine, 2001. 7(4). [DOI] [PubMed] [Google Scholar]
- 35.Herbst RS, Eckhardt SG, Kurzrock R, et al. , Phase I dose-escalation study of recombinant human Apo2L/TRAIL, a dual proapoptotic receptor agonist, in patients with advanced cancer. J Clin Oncol, 2010. 28(17): p. 2839–46. *Of importance due to description of results from phase I clinical trial testing of TRAIL. [DOI] [PubMed] [Google Scholar]
- 36.Soria JC, Mark Z, Zatloukal P, et al. , Randomized phase II study of dulanermin in combination with paclitaxel, carboplatin, and bevacizumab in advanced non-small-cell lung cancer. J Clin Oncol, 2011. 29(33): p. 4442–51. [DOI] [PubMed] [Google Scholar]
- 37.Kelley SK, HARRIS LA, XIE D, et al. , Preclinical Studies to Predict the Disposition of Apo2L/Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand in Humans: Characterization of in Vivo Efficacy, Pharmacokinetics, and Safety. The Journal of Pharmacology and Experimental Therapeutics, 2001. 299(1): p. 31–38. [PubMed] [Google Scholar]
- 38.Forero-Torres A, Varley KE, Abramson VG, et al. , TBCRC 019: A Phase II Trial of Nanoparticle Albumin-Bound Paclitaxel with or without the Anti-Death Receptor 5 Monoclonal Antibody Tigatuzumab in Patients with Triple-Negative Breast Cancer. Clin Cancer Res, 2015. 21(12): p. 2722–9. *Of importance due to demonstration of a signal in the clinic from anti-DR5 plus docetaxel in triple negative breast cancer patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ganten TM, Koschny R, Sykora J, et al. , Preclinical differentiation between apparently safe and potentially hepatotoxic applications of TRAIL either alone or in combination with chemotherapeutic drugs. Clin Cancer Res, 2006. 12(8): p. 2640–6. [DOI] [PubMed] [Google Scholar]
- 40.Berg D, Lehne M, Muller N, et al. , Enforced covalent trimerization increases the activity of the TNF ligand family members TRAIL and CD95L. Cell Death Differ, 2007. 14(12): p. 2021–34. [DOI] [PubMed] [Google Scholar]
- 41.Schneider B, Munkel S, Krippner-Heidenreich A, et al. , Potent antitumoral activity of TRAIL through generation of tumor-targeted single-chain fusion proteins. Cell Death Dis, 2010. 1: p. e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang F, Wang AP, and Yang SF, Antitumor activity of a novel recombinant mutant human tumor necrosis factor-related apoptosis-inducing ligand. Acta Pharmacol Sin, 2005. 26(11): p. 1373–81. [DOI] [PubMed] [Google Scholar]
- 43.Geng C, Hou J, Zhao Y, et al. , A multicenter, open-label phase II study of recombinant CPT (Circularly Permuted TRAIL) plus thalidomide in patients with relapsed and refractory multiple myeloma. Am J Hematol, 2014. 89(11): p. 1037–42. [DOI] [PubMed] [Google Scholar]
- 44.Chae SY, Kim TH, Park K, et al. , Improved antitumor activity and tumor targeting of NH(2)-terminal-specific PEGylated tumor necrosis factor-related apoptosis-inducing ligand. Mol Cancer Ther, 2010. 9(6): p. 1719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fang J, Nakamura H, and Maeda H, The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev, 2011. 63(3): p. 136–51. [DOI] [PubMed] [Google Scholar]
- 46.de Miguel D, Lemke J, Anel A, et al. , Onto better TRAILs for cancer treatment. Cell Death Differ, 2016. 23(5): p. 733–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Bruyn M, Bremer E, and Helfrich W, Antibody-based fusion proteins to target death receptors in cancer. Cancer Lett, 2013. 332(2): p. 175–83. [DOI] [PubMed] [Google Scholar]
- 48.Allen JE, Krigsfeld G, Patel L, et al. , Identification of TRAIL-inducing compounds highlights small molecule ONC201/TIC10 as a unique anti-cancer agent that activates the TRAIL pathway. Mol Cancer, 2015. 14: p. 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Allen JE, Krigsfeld G, Mayes PA, et al. , Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med, 2013. 5(171): p. 171ra17. *Of importance as the discovery of first-in-class TRAIL pathway inducer TIC10/ONC201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kline CLB and Pieter J JEAA. Heuvel Van den, Prabhu Varun V., Dicker David T., El-Deiry Wafik S., ONC201 kills solid tumor cells by triggering an integrated stress response dependent on ATF4 activation by specific eIF2a kinases. Science Signaling, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ishizawa J, Kojima K, Chachad D, et al. , ATF4 induction through an atypical integrated stress response to ONC201 triggers p53-independent apoptosis in hematological malignancies. Sci Signal, 2016. 9(415): p. ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prabhu VV, Allen JE, Dicker DT, et al. , Small-Molecule ONC201/TIC10 Targets Chemotherapy-Resistant Colorectal Cancer Stem-like Cells in an Akt/Foxo3a/TRAIL-Dependent Manner. Cancer Res, 2015. 75(7): p. 1423–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prabhu VV, Lulla AR, Madhukar NS, et al. , Cancer stem cell-related gene expression as a potential biomarker of response for first-in-class imipridone ONC201 in solid tumors. PLoS One, 2017. 12(8): p. e0180541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ralff MD, Kline CLB, Kucukkase OC, et al. , ONC201 demonstrates anti-tumor effects in both triple negative and non-triple negative breast cancers through TRAIL-dependent and TRAIL-independent mechanisms. Mol Cancer Ther, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karpel-Massler G, Ba M, Shu C, et al. , TIC10/ONC201 synergizes with Bcl-2/Bcl-xL inhibition in glioblastoma by suppression of Mcl-1 and its binding partners in vitro and in vivo. Oncotarget, 2015. 6(34): p. 36456–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner J, Kline CLB, Zhou L, et al. , Dose intensification of TRAIL-inducing ONC201 inhibits metastasis and promotes intratumoral NK cell recruitment. The Journal of Clinical Investigation, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stein MN, Bertino JR, Kaufman HL, et al. , First-in-human clinical trial of oral ONC201 in patients with refractory solid tumors. Clin Cancer Res, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wagner J, Kline CL, Ralff MD, et al. , Preclinical evaluation of the imipridone family, analogues of clinical stage anti-cancer small molecule ONC201, reveals potent anti-cancer effects of ONC212. Cell Cycle, 2017: p. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li B, Russell SJ, Compaan DM, et al. , Activation of the proapoptotic death receptor DR5 by oligomeric peptide and antibody agonists. J Mol Biol, 2006. 361(3): p. 522–36. [DOI] [PubMed] [Google Scholar]
- 60.Wang G, Wang X, Yu H, et al. , Small-molecule activation of the TRAIL receptor DR5 in human cancer cells. Nat Chem Biol, 2013. 9(2): p. 84–9. [DOI] [PubMed] [Google Scholar]
- 61.Allen JE, Ferrini R, Dicker DT, et al. , Targeting TRAIL death receptor 4 with trivalent DR4 Atrimer complexes. Mol Cancer Ther, 2012. 11(10): p. 2087–95. [DOI] [PubMed] [Google Scholar]
- 62.Crowder RN, Dicker DT, and El-Deiry WS, The Deubiquitinase Inhibitor PR-619 Sensitizes Normal Human Fibroblasts to Tumor Necrosis Factor-related Apoptosis-inducing Ligand (TRAIL)-mediated Cell Death. J Biol Chem, 2016. 291(11): p. 5960–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mirandola P, Ponti C, Gobbi G, et al. , Activated human NK and CD8+ T cells express both TNF-related apoptosis-inducing ligand (TRAIL) and TRAIL receptors but are resistant to TRAIL-mediated cytotoxicity. Blood, 2004. 104(8): p. 2418–24. [DOI] [PubMed] [Google Scholar]
- 64.Kim K, Fisher MJ, Xu Shi-Qiong, et al. , Molecular Determinants of Response to TRAIL in Killing of Normal and Cancer Cells. Clinical Cancer Research, 2000. 6: p. 335–346. [PubMed] [Google Scholar]
- 65.Horak P, Pils D, Haller G, et al. , Contribution of Epigenetic Silencing of Tumor Necrosis Factor–Related Apoptosis Inducing Ligand Receptor 1 (DR4) to TRAIL Resistance and Ovarian Cancer. Mol Cancer Res, 2005. 3(6). [DOI] [PubMed] [Google Scholar]
- 66.Hopkins-Donaldson S, Ziegler A, Kurtz S, et al. , Silencing of death receptor and caspase-8 expression in small cell lung carcinoma cell lines and tumors by DNA methylation. Cell Death Differ, 2003. [DOI] [PubMed] [Google Scholar]
- 67.Pai SI, Wu GS, Ozoren N, et al. , Rare loss-of-function mutation of a death receptor gene in head and neck cancer. Cancer Research, 1998. 58(16): p. 3513–3518. [PubMed] [Google Scholar]
- 68.McDonald ER 3rd, Chui PC, Martelli PF, et al. , Death domain mutagenesis of KILLER/DR5 reveals residues critical for apoptotic signaling. J Biol Chem, 2001. 276(18): p. 14939–45. [DOI] [PubMed] [Google Scholar]
- 69.Ozoren N, Fischer MJ, Liu CX, et al. , Homozygous deletion of the death receptor DR4 gene in a nasopharyngeal cancer cell line is associated with TRAIL resistance. Int J Oncol, 2000. 16(5): p. 917–925. [DOI] [PubMed] [Google Scholar]
- 70.Jin Z, McDonald ER 3rd, Dicker DT, et al. , Deficient tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) death receptor transport to the cell surface in human colon cancer cells selected for resistance to TRAIL-induced apoptosis. J Biol Chem, 2004. 279(34): p. 35829–39. [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y and Zhang B, TRAIL resistance of breast cancer cells is associated with constitutive endocytosis of death receptors 4 and 5. Mol Cancer Res, 2008. 6(12): p. 1861–71. [DOI] [PubMed] [Google Scholar]
- 72.Guan B, Ping Y, Clayman GL, et al. , Evidence That the Death Receptor DR4 Is a DNA Damage-Inducible, p53-Regulated Gene. Journal of Cellular Physiology, 2001. 188: p. 98–105. [DOI] [PubMed] [Google Scholar]
- 73.Takimoto R and El-Deiry W, Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene, 2000. 19: p. 1735–1743. [DOI] [PubMed] [Google Scholar]
- 74.Earel JK Jr., VanOosten RL, and Griffith TS, Histone deacetylase inhibitors modulate the sensitivity of tumor necrosis factor-related apoptosis-inducing ligand-resistant bladder tumor cells. Cancer Res, 2006. 66(1): p. 499–507. [DOI] [PubMed] [Google Scholar]
- 75.Singh TR, Shankar S, and Srivastava RK, HDAC inhibitors enhance the apoptosis-inducing potential of TRAIL in breast carcinoma. Oncogene, 2005. 24(29): p. 4609–23. [DOI] [PubMed] [Google Scholar]
- 76.Sayers TJ and Murphy WJ, Combining proteasome inhibition with TNF-related apoptosis-inducing ligand (Apo2L/TRAIL) for cancer therapy. Cancer Immunol Immunother, 2006. 55(1): p. 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jiang CC, Chen LH, Gillespie S, et al. , Tunicamycin sensitizes human melanoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by up-regulation of TRAIL-R2 via the unfolded protein response. Cancer Res, 2007. 67(12): p. 5880–8. [DOI] [PubMed] [Google Scholar]
- 78.Wagner KW, Punnoose EA, Januario T, et al. , Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med, 2007. 13(9): p. 1070–7. [DOI] [PubMed] [Google Scholar]
- 79.Teitz T and Kidd VJ, Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nature Medicine, 2000. 6(5). [DOI] [PubMed] [Google Scholar]
- 80.Irmler M, Thome M, Hahne M, et al. , Inhibition of death receptor signals by cellular FLIP. Nature, 1997. 388. [DOI] [PubMed] [Google Scholar]
- 81.Krueger A, Schmitz I, Baumann S, et al. , Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem, 2001. 276(23): p. 20633–40. [DOI] [PubMed] [Google Scholar]
- 82.Newsom-Davis T, Prieske S, and Walczak H, Is TRAIL the holy grail of cancer therapy? Apoptosis, 2009. 14(4): p. 607–23. [DOI] [PubMed] [Google Scholar]
- 83.Ricci MS, Jin Z, Dews M, et al. , Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol, 2004. 24(19): p. 8541–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kauh J, Fan S, Xia M, et al. , c-FLIP degradation mediates sensitization of pancreatic cancer cells to TRAIL-induced apoptosis by the histone deacetylase inhibitor LBH589. PLoS One, 2010. 5(4): p. e10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim Y, Suh N, Sporn M, et al. , An inducible pathway for degradation of FLIP protein sensitizes tumor cells to TRAIL-induced apoptosis. J Biol Chem, 2002. 277(25): p. 22320–9. [DOI] [PubMed] [Google Scholar]
- 86.Clohessy JG, Zhuang J, de Boer J, et al. , Mcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosis. J Biol Chem, 2006. 281(9): p. 5750–9. [DOI] [PubMed] [Google Scholar]
- 87.Munshi A, Pappas G, Honda T, et al. , TRAIL (APO-2L) induces apoptosis in human prostate cancer cells that is inhibitable by Bcl-2. Oncogene, 2001. 20: p. 3757–3765. [DOI] [PubMed] [Google Scholar]
- 88.LeBlanc H, LAWRENCE D, VARFOLOMEEV E, et al. , Tumor-cell resistance to death receptor–induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nature Medicine, 2002. 8(3). [DOI] [PubMed] [Google Scholar]
- 89.Huang S and Sinicrope FA, BH3 mimetic ABT-737 potentiates TRAIL-mediated apoptotic signaling by unsequestering Bim and Bak in human pancreatic cancer cells. Cancer Res, 2008. 68(8): p. 2944–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang G, Zhan Y, Wang H, et al. , ABT-263 sensitizes TRAIL-resistant hepatocarcinoma cells by downregulating the Bcl-2 family of anti-apoptotic protein. Cancer Chemother Pharmacol, 2012. 69(3): p. 799–805. [DOI] [PubMed] [Google Scholar]
- 91.Deveraux QL, Takahashi R, Salvesen GS, et al. , X-linked IAP is a direct inhibitor of cell-death proteases. Nature, 1997. 388. [DOI] [PubMed] [Google Scholar]
- 92.Du C, Fang M, Li Y, et al. , Smac, a Mitochondrial Protein that Promotes Cytochrome c–Dependent Caspase Activation by Eliminating IAP Inhibition. Cell, 2000. 102: p. 33–42. [DOI] [PubMed] [Google Scholar]
- 93.Verhagen AM, Ekert PG, Pakusch M, et al. , Identification of DIABLO, a Mammalian Protein that Promotes Apoptosis by Binding to and Antagonizing IAP Proteins. Cell, 2000. 102: p. 43–45. [DOI] [PubMed] [Google Scholar]
- 94.Fulda S, Wick W, Weller M, et al. , Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med, 2002. 8(8): p. 808–15. [DOI] [PubMed] [Google Scholar]
- 95.Lin L and Harran PG, A small molecule Smac mimic potentiates TRAIL- and TNF-mediated cell death. Science, 2004. 305(5689): p. 1471. [DOI] [PubMed] [Google Scholar]
- 96.Choi YE, Butterworth M, Malladi S, et al. , The E3 ubiquitin ligase cIAP1 binds and ubiquitinates caspase-3 and −7 via unique mechanisms at distinct steps in their processing. J Biol Chem, 2009. 284(19): p. 12772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang Y, Fang S, Jensen JP, et al. , Ubiquitin Protein Ligase Activity of IAPs and Their Degradation in Proteasomes in Response to Apoptotic Stimuli. Science, 2000. 288(5467): p. 874–877. [DOI] [PubMed] [Google Scholar]
- 98.Ricci MS, Kim SH, Ogi K, et al. , Reduction of TRAIL-induced Mcl-1 and cIAP2 by c-Myc or sorafenib sensitizes resistant human cancer cells to TRAIL-induced death. Cancer Cell, 2007. 12(1): p. 66–80. [DOI] [PubMed] [Google Scholar]
- 99.Katz SI, Zhou L, Chao G, et al. , Sorafenib inhibits ERK1/2 and MCL-1L phosphorylation levels resulting in caspase-independent cell death in malignant pleural mesothelioma. Cancer Biology & Therapy, 2014. 8(24): p. 2406–2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abdulghani J, Allen JE, and Dicker DT, Sorafenib sensitizes solid tumors to Apo2L/TRAIL and Apo2L/TRAIL receptor agonist antibodies by the Jak2-Stat3-Mcl1 axis. PLoS One, 2013. 8(9): p. e75414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zauli G and Di Pietro R, PI-3K/Akt and NF-κB/IκBα pathways are activated in Jurkat T cells in response to TRAIL treatment. Journal of Cellular Physiology, 2004. 202(3): p. 900–911. [DOI] [PubMed] [Google Scholar]
- 102.Samuels Y, Diaz LA Jr., Schmidt-Kittler O, et al. , Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell, 2005. 7(6): p. 561–73. [DOI] [PubMed] [Google Scholar]
- 103.Kandasamy K and Srivastava RK, Role of the Phosphatidylinositol 3 -Kinase/PTEN/Akt Kinase Pathway in Tumor Necrosis Factor-related Apoptosis-inducing Ligand-induced Apoptosis in Non-Small Cell Lung Cancer Cells. Cancer Research, 2002. 62: p. 4929–4937. [PubMed] [Google Scholar]
- 104.Kim S, Kang J, Qiao J, et al. , Phosphatidylinositol 3-kinase inhibition down-regulates survivin and facilitates TRAIL-mediated apoptosis in neuroblastomas. Journal of Pediatric Surgery, 2004. 39(4): p. 516–521. [DOI] [PubMed] [Google Scholar]
- 105.Puduvalli VK, Sampath D, Bruner JM, et al. , TRAIL-induced apoptosis in gliomas is enhanced b∗y Akt-inhibition and is independent of JNK activation. Apoptosis, 2005. 10: p. 233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Panner A, James CD, Berger MS, et al. , mTOR controls FLIPS translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol Cell Biol, 2005. 25(20): p. 8809–23. [DOI] [PMC free article] [PubMed] [Google Scholar]


