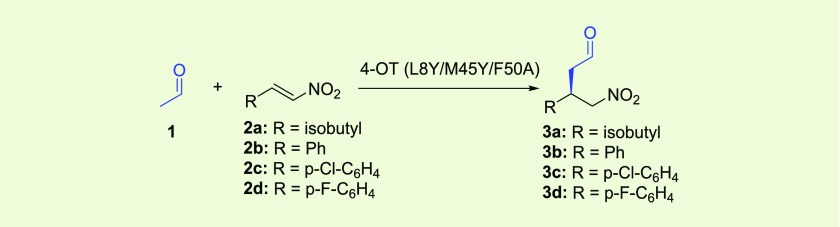
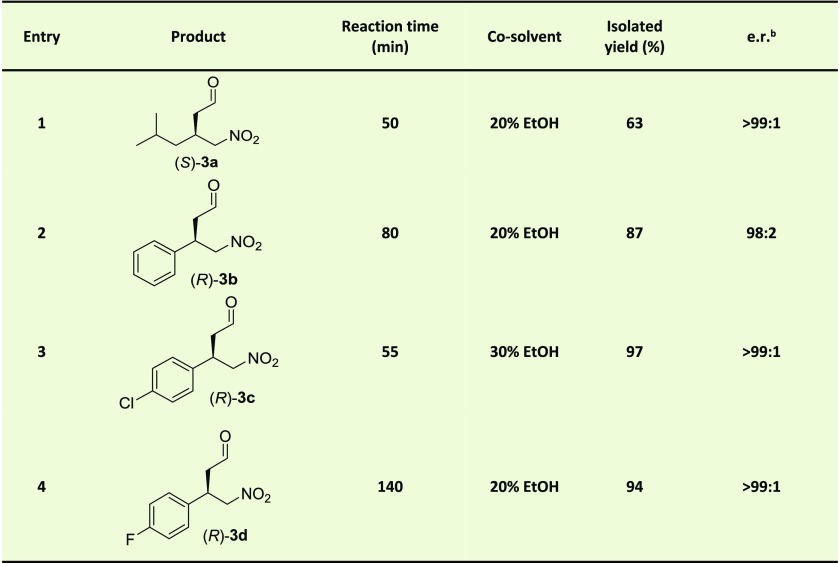
Table 1. 4-OT L8Y/M45Y/F50A Catalyzed Michael-Type Additions of 1 to 2a–d Yielding 3a–da.
Assay conditions: reaction mixtures consisted of 150 mM 1 (synthesis of 3a) or 50 mM 1 (synthesis of 3b–d), 3 mM 2a, 1.3 mM 2c or 2 mM 2b and 2d in 20 mM sodium phosphate buffer, pH 6.5. The reaction volume was 12.8 mL (synthesis of 3a) or 60 mL (synthesis of 3b–d). 1.4 mol % of 4-OT (compared to concentration nitroalkene) was used, except for entry 1 (2.5 mol %) and entry 3 (2.15 mol %).
For 3a, the e.r. was determined by GC with a chiral stationary phase. For 3b and 3c, the enzymatic product was first converted into the corresponding ethylene glycol acetal, of which the e.r. was determined by HPLC with a chiral stationary phase. For 3d, the e.r. was directly determined by HPLC with a chiral stationary phase. The absolute configuration was determined by comparison to literature data.17,25