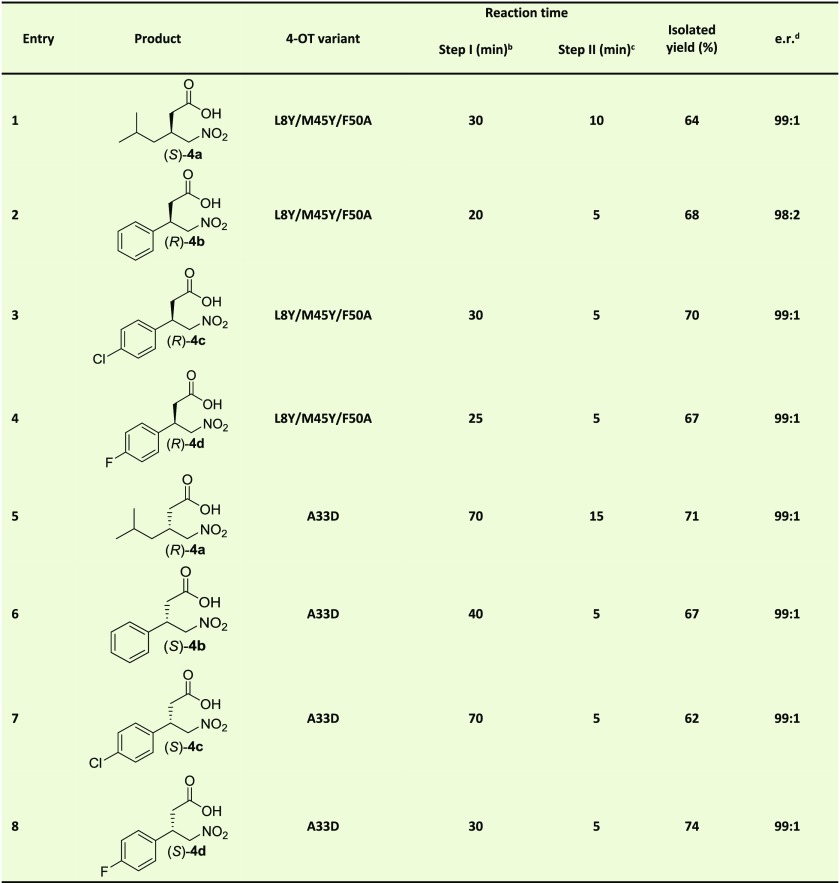
Table 2. One-Pot Two-Step Biocatalytic Enantioselective Synthesis of γ-Nitrobutyric Acids 4a–da.
Michael-type addition of 1 to 2a–d catalyzed by 4-OT L8Y/M45Y/F50A or 4-OT A33D to form either S-3a and R-3b–d or R-3a and S-3b–d respectively, followed by aldehyde oxidation catalyzed by PRO-ALDH(003), using PRO-NOX(009) for cofactor recycling, to form either S-4a and R-4b–d or R-4a and S-4b–d. The reaction mixtures consisted of 50 mM 1, 3 mM 2a or 4 mM 2b–d in 100 mM sodium phosphate buffer pH 7.3 and 10% (v/v) ethanol. Five mol % of 4-OT (compared to concentration nitroalkene) was used, except for entry 6 (1.5 mol %) and entry 7 (3 mol %). PRO-ALDH(003) was added to a final concentration of 0.5 mg/mL, PRO-NOX(009) was added to a final concentration of 1 mg/mL. The concentration NAD+ was 8 mM (4a) or 10 mM (4b–d). The amounts of applied cofactor were adjusted such that short reaction times and high conversions were achieved.
Monitored by UV spectroscopy.
Monitored by HPLC.
Products 4a–d were esterified and the e.r. was determined by GC or HPLC with a chiral stationary phase.