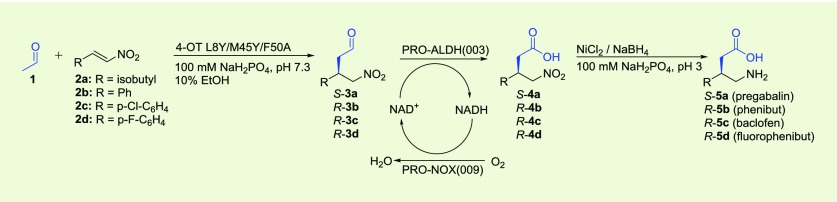
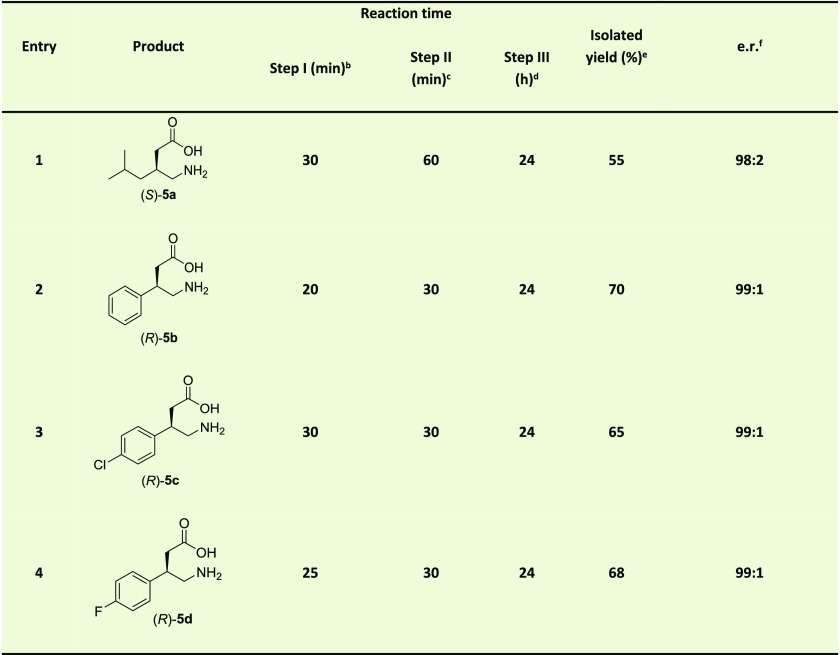
Table 3. One-Pot Three-Step Chemoenzymatic Cascade Synthesis of Pharmaceuticals 5a–da.
Michael-type addition of 1 to 2a–d catalyzed by 4-OT L8Y/M45Y/F50A to form S-3a and R-3b–d, followed by aldehyde oxidation catalyzed by PRO-ALDH(003), using PRO-NOX(009) for cofactor recycling, to form S-4a and R-4b–d, followed by nitro reduction catalyzed by nickel boride to form S-5a and R-5b–d. The reaction mixtures consisted of 50 mM 1, 3 mM 2a or 4 mM 2b–d in 100 mM sodium phosphate buffer pH 7.3 and 10% (v/v) ethanol. Five mol % of 4-OT (compared to concentration of nitroalkene) was used; PRO-ALDH(003) was added to a final concentration of 0.5 mg/mL; PRO-NOX(009) was added to a final concentration of 1 mg/mL; 0.5 mM of NAD+ was added; 40 mM of NiCl2 and 40 mM of NaBH4 were used. Because of the poor stability of nickel boride in aqueous buffer, a 10-fold excess of this reagent (40 mM versus 3–4 mM 2a–d) is required to achieve high conversion.
Monitored by UV spectroscopy.
Monitored by HPLC.
Monitored by TLC.
Purified by cation exchange chromatography.
Products 5a–d were derivatized using Nα-(2,4-dinitro-5-fluorophenyl)-l-valinamide, and the e.r. of the corresponding diastereomers was determined by HPLC with an achiral stationary phase. Notably, the e.r. of product 5a is likely to be 99:1; however, because a minor contaminant from the derivatizing agent has the same retention time as the R enantiomer of product 5a, the e.r. for product 5a is cautiously reported as 98:2, consistent with the observed peaks in the HPLC chromatogram.