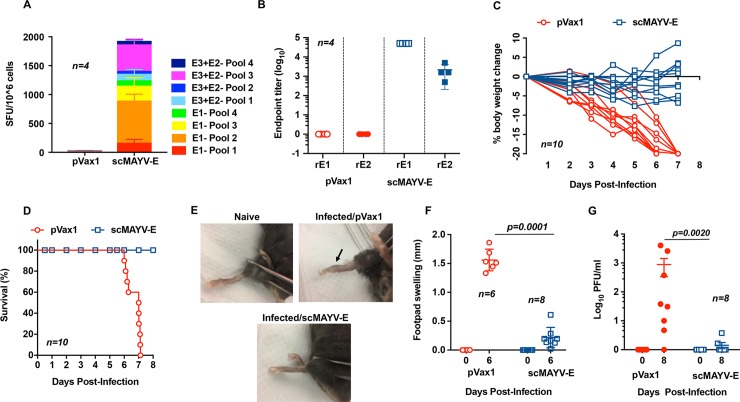
Fig 6. scMAYV-E protects immunized mice from MAYV challenge.
IFNAR-/- mice aged 4–6 weeks old were immunized twice, two weeks apart with pVax1 or scMAYV-E using EP-enhanced i.m. injection. Groups of mice for immunogenicity studies were euthanized one week after the final immunization. (A) Evaluation of cellular responses in vaccinated IFNAR-/- mice. IFN-γ ELISpot of pVax1 or scMAYV-E immunized splenocytes is shown (n = 4). (B) Evaluation of humoral responses in vaccinated IFNAR-/- mice one week after the second immunization prior to viral challenge. Endpoint titers for rE1-IgG and rE2-IgG were evaluated for pVax1 or scMAYV-E immune sera (n = 4). Mice were challenged intraperitoneally (i.p.) one week after the second immunization (day 21) with 102 PFU of MAYV TRVL 15537. All mice were observed daily for clinical signs of disease up to 8 days post challenge. (C) Percent change in bodyweight from day 0 in individual immunized mice post challenge (n = 10); p = 0.0115 and (D) a Kaplan-Meier survival curve of scMAYV-E or pVax1 immunized mice post-MAYV challenge (n = 10; survival (%): scMAYV-E = 100, pVax1 = 0). (E) Representative pictures of rear footpad of uninfected mouse (naive), pVax1 immunized mouse (Infected/pVax1), and scMAYV-E immunized mouse (Infected/scMAYV-E) at 6 days post challenge. (F) Quantification of rear footpad size as measured by a caliper on day 6 post-MAYV challenge (n = 6 pVax1; n = 8 scMAYV-E). (G) MAYV PFU/ml in sera collected from pVax1 and scMAYV-E immunized mice at day 6 post MAYV challenge (n = 8).