Figure 3.
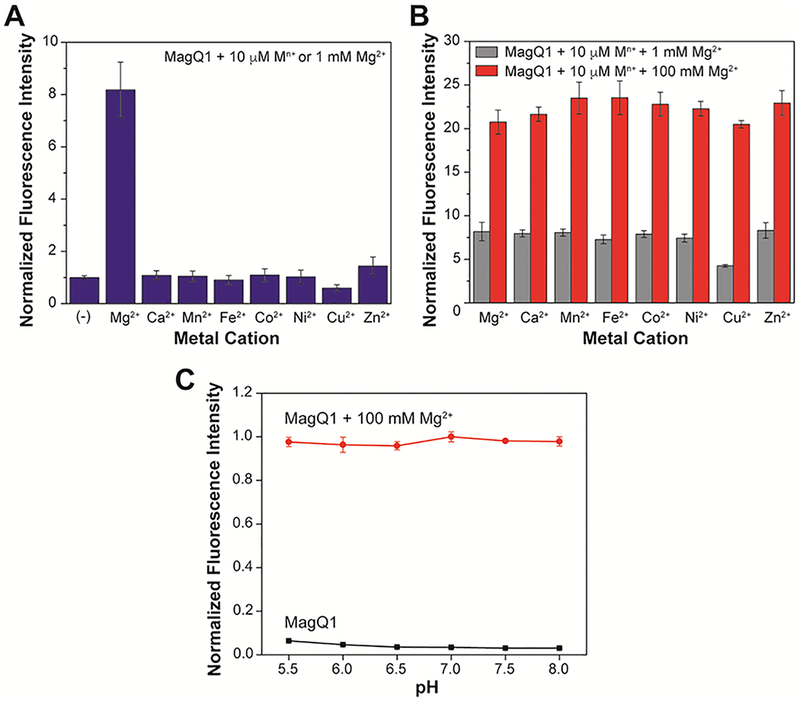
(A) Fluorescence response of 1.0 μM MagQ1 to physiological concentration of Mg2+ (1 mM) or to other divalent metal ions (10 μM) in aqueous buffer at 25 °C. (B) Fluorescence response of 1.0 μM MagQ1 to 1 mM or 100 mM of Mg2+ in the presence of competing divalent cations, showing the selectivity of the detection in 50 mM PIPES, 100 mM KCl, pH 7.0 buffer. (C) Fluorescence emission of a 1 μM solution of MagQ1 in aqueous buffer at pH ranging from 5.5 to 8.0 at 25 °C, in the absence (black squares) or presence (red circles) of 100 mM Mg2+. Excitation wavelength λex = 600 nm; emission wavelength λem = 635 nm. Error bars correspond to standard deviations on measurements conducted in triplicate.
